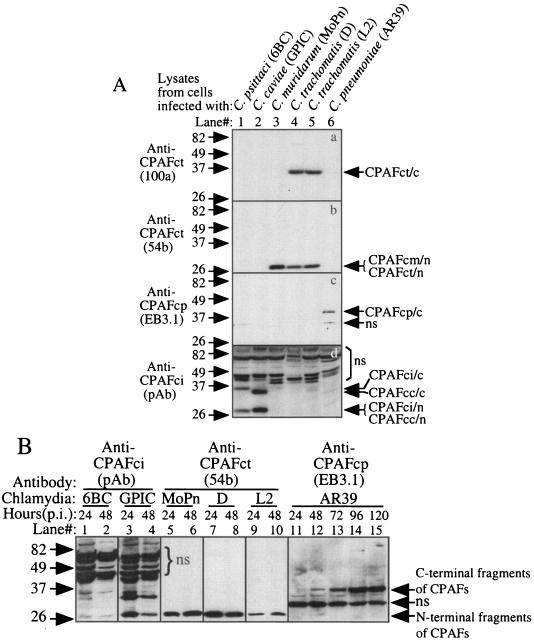
FIG. 2.
Detection of endogenous CPAF proteins expressed in chlamydia-infected cells. (A) HeLa cells infected with the various chlamydial organisms indicated at the top of the figure were lysed with an MLB lysis buffer 48 h after infection. The lysates were used as antigens to screen the various anti-CPAF antibodies on a Western blot. Please note that the monoclonal antibodies 100a, 54b, and EB3.1 recognized the 35-kDa C-terminal fragment of C. trachomatis CPAF (CPAFct/c), the 29-kDa N-terminal fragments of C. trachomatis and C. muridarum CPAF (CPAFct/n and CPAFcm/n), and the 35-kDa C-terminal fragment of C. pneumoniae CPAF (CPAFcp/c), respectively. The polyclonal antibody (pAb) raised against CPAF from C. psittaci 6BC (CPAFci) recognized both the C- and N-terminal fragments of CPAF from C. psittaci (CPAFci/c and CPAFci/n) and C. caviae (CPAFcc/c and CPAFcc/n). ns, nonspecific binding. (B) The antibodies listed above except 100a were used to monitor CPAF expression in cells infected with various chlamydia strains for various periods of time, as indicated at the top of the figure, on a Western blot. The infected cell lysates were prepared in the way described above and were loaded with 1 μl per lane after the total amounts of proteins in each sample were equalized.