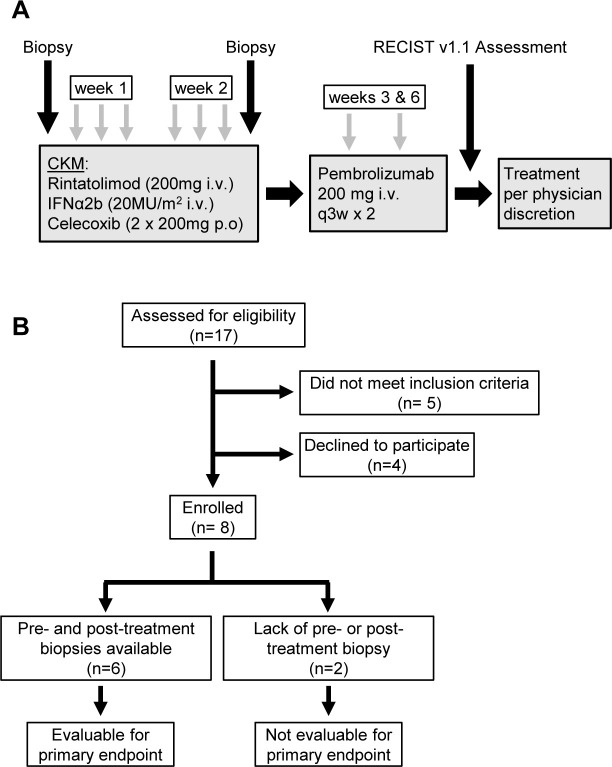
Figure 1.
Study design. (A) Systemic chemokine modulation (CKM) was given as six doses over 2 weeks (days 0, 1, 2, 7, 8, and 9). Each daily CKM consisted of intravenous IFN-α2b (20 MU/m2; over 30 min) followed by rintatolimod (200 mg; over 2.5 hours) and oral celecoxib (two doses of 200 mg; 12 hours apart). Tumor biopsies were performed before and after CKM, with the second biopsy performed 1–3 days post-CKM. As a follow up to CKM, patients received two cycles of pembrolizumab (200 mg intravenous), starting 3–8 days after completion of the second course of CKM. Following completion of pembrolizumab, tumors were assessed using RECIST V.1.1 guidelines. Patients continued treatment per physician discretion following completion of the trial. (B) Consolidated Standards of Reporting Trials flow diagram depicting screening, enrollment, and follow up of participants in the trial. The trial enrolled eight patients, six of whom were evaluable for the primary endpoint of CD8α changes in pre-CKM and post-CKM biopsies. IFN, interferon; RECIST, Response Evaluation Criteria for Solid Tumors.