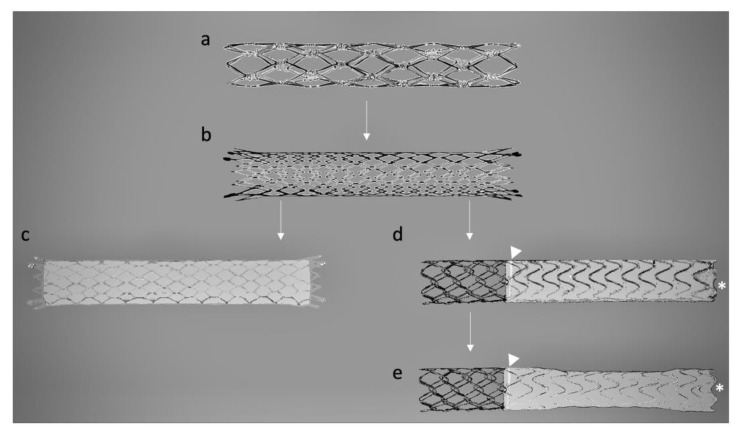
Figure 2.
Schematic illustration of the evolution of TIPS devices from the BMSs era to the ePTFE-SGs with controlled expansion technology. (a) Palmaz® (Cordis, Miami, FL, USA), a balloon-expandable stainless steel stent; (b) Wallstent® (Boston Scientific, Marlborough, MA, USA), a self-expandable BMS made of nickel–cobalt–titanium–steel alloy, with a braided closed-cell design; (c) Fluency® (Angiomed GmbH, a subsidiary of C.R. Bard, Inc., Karlsruhe, Germany), a fully covered grid-like stent composed of a biocompatible nickel–titanium alloy, wrapped internally and externally in ePTFE, with 2 mm of bare regions and two radiopaque titanium markers for imaging purposes at both extremities; (d) VIATORR® (W.L. Gore & Associates in Phoenix, AZ, USA), a self-expandable dedicated nitinol stent made of a 4 to 8 cm portion covered with ePTFE on the inside and a bare 2 cm long PV portion. A circumferential radiopaque gold band (arrowhead) marks the transition between the covered and uncovered portions and an additional radiopaque gold marker (*) is embedded at the trailing edge of the device; (e) VIATORR® Endoprosthesis with Controlled Expansion (W.L. Gore & Associates, Phoenix, AZ, USA), analogous to the VTS with an additional outer constraining balloon-expandable sleeve on the lined region of the stent. Abbreviations: BMSs, bare metal stents; ePTFE, expanded polytetrafluoroethylene; PV, portal vein; TIPS, transjugular intrahepatic portosystemic shunt.