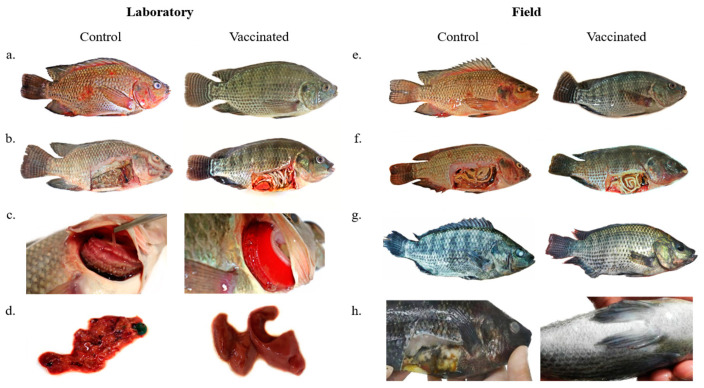
Figure 4.
(a) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting body and fin hemorrhages and ulcerative lesions. Fish from the vaccinated group showing no external clinical signs after experimental infection. (b) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting skin pallor, yellowish fluid in the visceral cavity, and organ deterioration. Fish from the vaccinated group showing no internal clinical signs after experimental infection. (c) Control group fish after experimental infection with A. hydrophila and S. agalactiae presenting excess mucus, necrosis, and pallor of the gills. Fish gills of the vaccinated group with normal appearance and color after experimental infection. (d) Fish liver from the control group after experimental infection with A. hydrophila and S. agalactiae showing deterioration and hemorrhage. Fish liver of the vaccinated group showing normal appearance and color after experimental infection. (e) Control group fish presenting body and fin hemorrhages. Vaccinated group fish showing no external clinical signs. (f) Control group fish presenting skin pallor, yellowish fluid in the visceral cavity, and organ deterioration. Vaccinated group fish showing no internal clinical signs. (g) Control group fish presenting tail rot and corneal opacity. Vaccinated group fish showing weight gain and no clinical signs. (h) Control group fish showing skin darkening, corneal opacity, and internal organ deterioration.