Abstract
Anal cancer incidence is higher in persons living with HIV/AIDS (PLWHA) than in the general population. Participation of PLWHA in anal cancer clinical trials (CTs) is essential; Hispanic PLWHA are underrepresented in CTs. We conducted a behavioral CT among 305 PLWHA in Puerto Rico to measure the efficacy of an educational video in increasing calls and screening into an anal cancer CT. Participants received printed educational materials on anal cancer and CTs; the intervention group also received an educational video. Outcome assessment based on follow-up interviews showed that printed materials increased awareness about CTs and high-resolution anoscopy (HRA), and willingness to participate in an anal cancer CT in both groups. However, the addition of the video increased the likelihood of participants to call the CT for orientation (RRadjusted=1.66, 95%CI: 1.00–2.76; p=0.05) and pre-screening evaluation (RRadjusted=1.70, 95%CI: 0.95–3.03; p=0.07). This intervention could help increase participation of Hispanics into anal cancer-related CTs.
Keywords: Hispanics, anal cancer, clinical trials, behavioral intervention, HIV/AIDS
Resumen
El cáncer anal es más común en personas con VIH/SIDA que en la población general. La participación de estas personas en ensayos clínicos (ECs) es esencial; los hispanos están sub-representados en los ECs. Realizamos un EC en 305 personas con VIH/SIDA en Puerto Rico, para medir la eficacia de un video educativo en aumentar las llamadas y el cernimiento a un EC de cáncer anal. Los participantes recibieron material impreso sobre cáncer anal y ECs; el grupo de intervención también observό el video educativo. Los resultados mostraron que los materiales impresos aumentaron la concienciación sobre ECs y la anoscopía, y la disposición de participar en un EC de cáncer anal. Sin embargo, el video educativo aumentό la posibilidad de que los participantes llamaran para orientación (RRajustado=1.66, 95%CI: 1.00–2.76; p=0.05) y evaluación (RRajustado=1.70, 95%CI: 0.95–3.03; p=0.07) para un ECs de cáncer anal. Esta intervención puede ayudar a aumentar la participación de Hispanos en ECs de cáncer anal.
Introduction
The burden of anal cancer is much higher in persons living with HIV/AIDS (PLWHA) than the general population (1). The lack of scientific evidence that supports the efficacy of anal screening and treatment to reduce the incidence and mortality from anal cancer, has limited the development of effective clinical guidelines in this area. There is a critical need for these guidelines (2), as these are essential for cancer control strategies.
The Anal Cancer/HSIL Outcomes Research (ANCHOR) study (https://anchorstudy.org/; National Cancer Institute protocol AMC-A01) is an ongoing Phase III multicenter clinical trial (CT) whose primary aim is to determine whether treating anal high-grade squamous intraepithelial lesions (HSILs) is effective in reducing anal cancer incidence in adults with HIV aged 35 years and older compared with monitoring lesions without treatment. The ANCHOR Study currently has 15 clinical sites in the US, including the unincorporated US territory of Puerto Rico (PR). Trials such as the ANCHOR Study need PLWHA who are willing to participate in them in order to be successful. Hence, there is a necessity to develop behavioral interventions that will target PLWHA to increase awareness, participation, recruitment and retention of this population in CTs for anal cancer.
Limited studies have tested interventions that address challenges in CT enrollment (3). There are recommendations to developed and tested culturally sensitive educational interventions as a strategy to increase the participation of racial/ethnic minorities in CTs (3). This is of particular relevance for Hispanics, as historically, studies in the US have shown their low participation in CTs (4,5), particularly those who do not speak English (4,6,7). Hispanics are also less likely than Whites to report having tried or participated in HIV-related trials (8). Most common reasons for Hispanics low participation are mistrust, language barriers and lack of knowledge of CTs (6). Health education and literacy, as well as decision aids, are essential for appropriate decision making of patients in the health care setting (9).
Educational strategies for patients include verbal and written instructions and materials, as well as audio-visual interventions. Advantages of audiovisual materials include that this communication strategy is familiar, that they are entertaining and are a good resource for persons with limited literacy (10). Systematic reviews have examined the effectiveness of videos in modifying health behaviors within controlled CTs, showing varied results in terms of patient willingness to participate into cancer trials (11,12). Areas in which videos have shown effectiveness include increased cancer screening and HIV testing, improving informed consent comprehension, and adherence to treatment (13–15). Although educational videos for HIV/AIDS exist (http://www.aidsvideos.org/, https://www.helpstopthevirus.com/hiv-education), most are in English, and limited research exists on their effectiveness for PLWHA.
Despite the current interest in CTs of anal cancer, no previous study has evaluated the impact of educational videos in enrollment in such trials. Studies that assess the effectiveness of interventions that increase CT willingness and participation among PLWHA are needed. This information is necessary to create effective recruitment strategies and advance anal cancer screening and treatment research, as well as guidelines for this population. The primary aim of this behavioral site randomized controlled trial (RCT) was to measure the efficacy of an educational video in increasing calls and screening rates into an anal cancer CT (the ANCHOR study). Secondary aims included testing the efficacy of the intervention in increasing awareness of CTs and high-resolution anoscopy (HRA), knowledge about CT, and willingness to participate in a trial that used HRA.
Methods
Study Design.
A clinic-based behavioral site randomized controlled trial with two-arms was conducted in all nine immunology/sexually transmitted infections clinics (n=9) of the PR Department of Health.
Randomization of clinics and inclusion criteria.
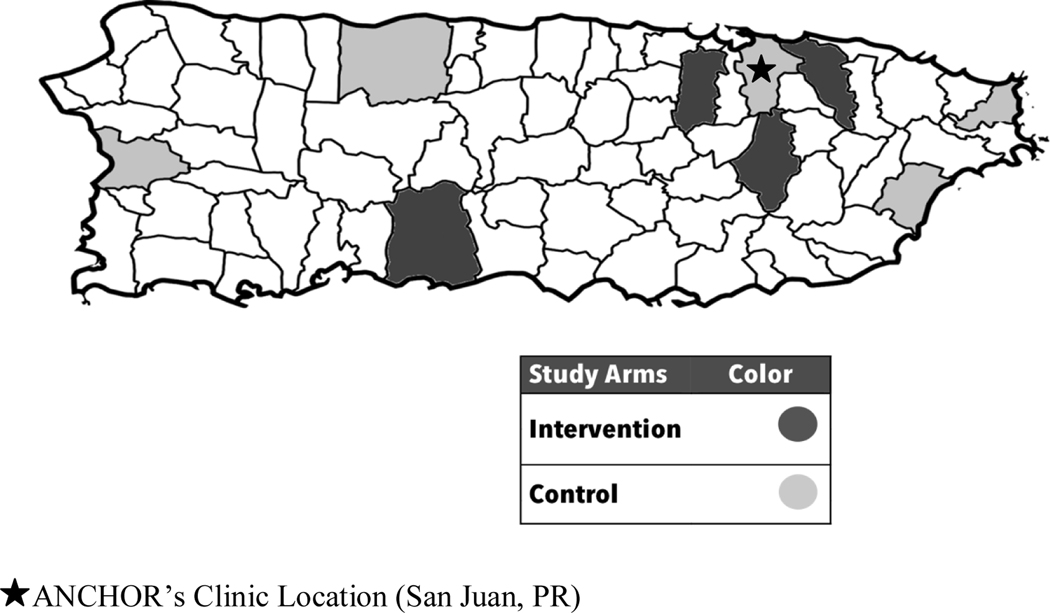
To take into account the distribution of HIV cases across PR, the clinics were paired according to the number of HIV-infected patients and the geographical distance between the clinics. The sample size of each pair was proportional to the number of patients attended in 2015. The clinics were randomly allocated into one of the study arms (Figure 1). Five clinics composed the control arm and four the intervention arm. In each pair, the sample size of women and men was proportional to the number of women and men with HIV in PR (1 woman: 3 men). Clinic randomization minimized contamination of the groups with respect to the intervention.
Figure 1.
Randomization of Clinics in the Intervention and Control Arms
Eligible PLWHA were:
HIV+ men and women aged ≥35 years, who had never been diagnosed with cancer, had never participated in a cancer-related CT, with no plans of moving out of PR in the next six months, and who demonstrated to be mentally capable of completing the interview.
Participant recruitment and data collection procedures.
This study was approved by the Institutional Review Board (IRB) of the University of Puerto Rico, Medical Sciences Campus. A series of strategies were used for the study recruitment. PLWHA who had a scheduled appointment in any of the collaborating clinics were approached within the clinics using a study flyer. Clinic nurses helped research staff to identify potential eligible patients. Group orientations of patients within the clinics’ waiting room were also done. If the patient was interested, they were given additional one-to-one information about the study. If the patient agreed to participate they were screened to corroborate their eligibility, in a separate room. If eligible, patients were provided a written informed consent, a baseline face-to-face computer-based interview, and an initial contact survey. After the survey, participants whose clinic was allocated in the intervention arm received a 5-minute educational video intervention. Meanwhile, participants from both the intervention and control arms received four different educational materials, which included educational printed materials developed by our team on: (1) anal cancer, (2) CTs, (3) the ANCHOR Study, and (4) the Anal Neoplasia Clinic (clinical site of the ANCHOR study in PR, located in San Juan, PR [Figure 1]). Afterwards, they received a compensation of $20 for their time and effort, and proceeded to their regularly scheduled clinical visit.
Two follow-up interviews were completed by participants either through a phone call (just in the first follow-up) or within the clinic, if they had a follow-up clinical visit scheduled. The first follow-up interview occurred 2–8 weeks from recruitment, while the second follow-up occurred 3–9 months post recruitment. Participants were given $10 for completion of one follow-up and $20 for completion of two follow-ups. Participants who completed follow-up interview by phone were asked for their postal address, to send a gift card with the corresponding amount via certified mail. Participant recruitment (baseline visits) started on April 2016 until April 2017, and follow-interviews were conducted until July 2017. A tablet with the REDCap’s Mobile Offline Application was used for data collection purposes in the field for the baseline visit, while follow-up interviews where done in person or through the phone, documented in paper, and then entered into the REPCap server (16).
Video Description.
Three organizations collaboratively developed an educational video to enhance anal cancer CT participation in 2015. These included the University of Puerto Rico Comprehensive Cancer Center, The Medical Sciences Campus of the University of Puerto Rico and University of California, Davis Campus. The theoretical basis that our educational video used is the Social Cognitive Theory (17), which frames response to health communications based on the dynamic interplay of the individual’s cognitive, social, and emotional state; environment (e.g., social and physical); and behavior (17). In the case of our videotape, we chose a caring and trusted environment (HIV clinics); accommodated the perceived mindset knowledge of anal cancer CTs and ways by which HIV positive patients could contribute to expanding the knowledge of controlling this cancer; social—transmittal of information in an easily understood visual (video) and audio (in Spanish) manner; to the recruitment and rationale for the desired behavior (i.e., call for information).
This 5-minute video was developed in both Spanish and English. From physicians and patients’ perspectives, and using real people who acted out the scenes, the video highlights the importance of volunteering in CTs to help find better ways to prevent, detect and treat cancer for PLWHA, especially anal cancer. It explains that an anal cancer CT may require a HRA, and explains how the test is performed. The video also underscores the importance of talking to doctors about participating in CTs and contribute to find new cures and treatments for cancers in PLWHA. Also, the video explains that participation is voluntary, and that quitting can be done at any time. The script and the video was presented to members of our local Community Advisory Board for feedback before its production to take into account beliefs, norms, and practices of the people to be served, making the video culturally sensitive (18); some changes were incorporated as a result of their input, these included the inclusion of video images from Puerto Rico, and the use of words in Spanish common to Spanish speakers from Puerto Rico. The Spanish version of the video was used within this study, as the primary language used in Puerto Rico is Spanish.
Printed educational material description.
Educational materials previously generated by our research group were used; although these had been developed in English and Spanish, the Spanish versions were used in this study. These included brochures on: (1) CTs for persons with HIV, (2) HPV and anal cancer in women (given to female participants), and (3) HPV and anal cancer in men (given to male participants). The material on CTs included information on basic elements of a trial, including advantages, risks, and confidentiality. It also included key questions to ask when seeking information to participate in a CT. HPV information included: what HPV is, how it is transmitted, its relationship with anal cancer and vaccination; anal cancer information included: symptoms, risk factors, prevention, and detection. The ANCHOR study flyer included information regarding the purpose of the study, requirements for participation, a brief explanation of the study, and contact information (name, email and phone number of study coordinator) of the ANCHOR trial in PR. Additionally, the brochure of the Anal Neoplasia Clinic included a summary of the services offered in the clinic, and contact information (telephone number and facebook page).
Data Collection Instruments.
The baseline survey collected information regarding socio-demographic characteristics, healthcare access, physician-patient relationship, lifestyle practices and medical history. In addition, the baseline and follow-up surveys collected information regarding willingness, attitudes, and knowledge about CTs and HRA, and calls made to the ANCHOR trial. In addition, the pre-screening evaluation log of the ANCHOR’s study (Puerto Rico site) was used to validate whether participants completed the telephone-based pre-screening evaluation of ANCHOR the ANCHOR trial. This was possible given an established collaboration with the ANCHOR study PR site.
Study Outcomes.
The two primary study outcomes were: (1) patient called to receive orientation about ANCHOR study, and (2) patient completion of a telephone-based interview for pre-screening evaluation of the ANCHOR study. The pre-screening evaluation includes self-reported information on eligibility criteria, including date of birth, gender (at birth), HIV status, history of HPV-related anogenital cancers, and history of treatment for anal HSIL. Information on calls to ANCHOR study was obtained from the follow-up surveys of the current study. If a person was contacted in any of these two follow-ups, their response to this question was used for this assessment. Four secondary outcomes were evaluated. These included awareness of CTs and HRA (yes/no), which were assessed by the following questions: Before today, had you ever heard of CTs and Before today, had you ever heard of HRA?. We also assessed willingness (yes/no) to participate in a CT that used a HRA. In addition, knowledge about CT was assessed with the following question: According to your knowledge, please tell me which of the following fourth definitions best describes a CT: 1) a project in a clinic, 2) a test in the doctor’s office, 3) a research project in which some patients are chosen to test new treatments while others patients received different medications or none at all, or 4) a group of medicine students. The selection of alternative 3 was considered a correct response to this question. These outcomes were all assessed based on responses to the baseline and the first follow-up interview.
Other covariates.
Socio-demographic and behavioral variables included: age at recruitment (years), age at HIV diagnosis, years living with HIV, gender (male vs. female), place of birth (PR vs. other), marital status (never married vs. other), education level (≤ 12 years vs. >12 years), annual income (< $15,000 vs. ≥ $15,000), employment status (employed vs. unemployed), health insurance (government health insurance vs. other), sexual orientation (homosexual or bisexual vs. heterosexual), and adherence to antiretroviral therapy (ART) (yes vs.no). A 1 to 5 Likert scale was used to evaluate items related to perceived susceptibility and severity of anal cancer, as well as perceived benefits of CTs. Items for perceived susceptibility to anal cancer included: (1) I have greater possibilities of developing anal cancer in comparison with other people of my same age, and (2) I am very worried about developing anal cancer. For perceived severity items included: (1) Anal cancer is a hopeless disease, and (2) Being diagnosed with anal cancer scares me. Perceived benefits of anal cancer CTs was assessed by the following items: (1) Participating in CTs to detect anal cancer at earlier stages will help to protect my health, and (2) CTs help people living with HIV to fight against cancer.
Statistical analysis.
Socio-demographic and behavioral characteristics were compared by intervention status using Chi-square test for categorical variables and t-test or Wilcoxon’s Rank Sum for continuous variables. We compared participants with complete follow-up and participants who were lost to follow-up to see if there were differences in socio-demographic and behavioral characteristics (data not shown; no differences (p>0.05) were observed between these groups). Logistic regression models were used to estimate risk ratios (RRs) for the efficacy of the intervention in the primary outcomes. We dichotomized the primary outcomes, (1) called to receive orientation about ANCHOR (yes vs.no) and (2) completed ANCHOR screening evaluation (yes vs.no), based on whether the participant had completed these outcomes regardless of the time they made the call. Even though intervention and control groups were similar with respect to age and sex, we adjusted multivariate models for these covariates. In addition, we adjusted for the item that assessed if the participant was scared of an anal cancer diagnosis, given differences in mean scores of this variable in the intervention and control arms. Descriptive statistics were used to describe the main reasons reported during the first follow-up interview for not having called the ANCHOR Study.
As secondary analyses, we estimated the crude RRs for awareness of CTs and HRA, knowledge of CTs, and willingness to participate in a CT that uses HRA at 2 weeks, in order to determine immediate effect of the intervention. We adjusted these analyses for the outcome variable at baseline (i.e. willingness to participate in a CT that uses HRA), sex, age, and being scared of an anal cancer diagnosis. We also used adjusted mixed regression models to estimate the efficacy of the intervention on secondary outcomes changes over time (change from baseline to first follow-up). Descriptive statistics were used to describe the main reasons reported during the first follow-up interview for not having called to receive orientation about the ANCHOR study. All statistical analyses were performed using STATA/SE (version 14.0).
Results
Participant recruitment.
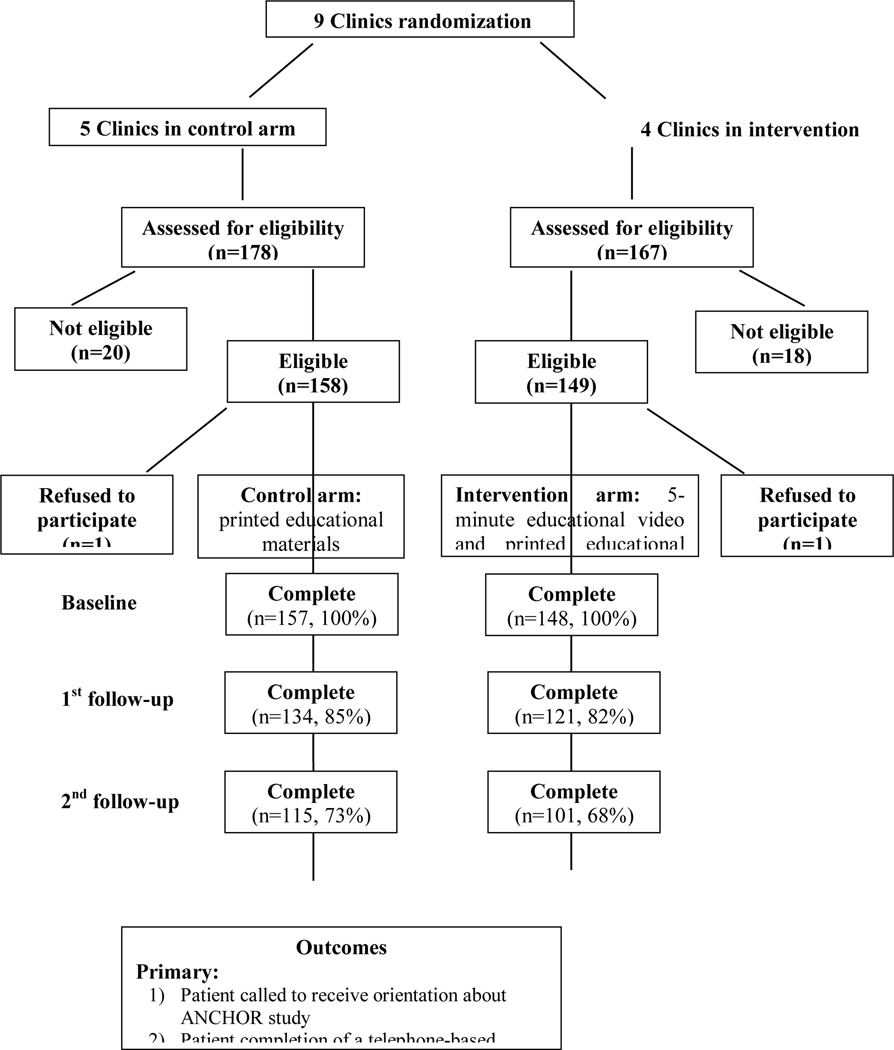
Three hundred forty-five people were assessed for eligibility, in which 307 were eligible (158 in the control arm and 149 in the intervention arm). Among eligible participants who were screened for eligibility into the study, two participants refused to be part of the study, for an overall response rate of 99%, yielding a final sample size (n=305; 157 persons in the control and 148 persons in the intervention group).
Baseline characteristics.
None of the demographic characteristics differed by group (p > 0.05) (Table I). Mean age was a little over 50 years for both groups, while around two-thirds had an education of high school or less. Over 50% of participants had heard about a CT before the study, while around 20% had previously participated in a non-cancer related CT. Regarding the perception of study participants about anal cancer and CTs (Table II), a significant difference between groups was observed at baseline for the variable being diagnosed with anal cancer scares me (p < 0.05), with the intervention group having the lowest mean (4.09 ± 1.50 compared to 4.38 ± 1.31 for the control group). No significant differences between study’s groups were observed for other characteristics (p > 0.05).
Table I.
Baseline characteristics of participants by study group (n=305)*
| Characteristics | Control (n=157) N (%) | Intervention (n=148) N (%) |
|---|---|---|
| Age at baseline interview (years) (mean, SD) | 52.13, 8.94 | 53.32, 9.29 |
| Age at HIV-AIDS diagnosis (years) (mean, SD) | 36.89, 9.73 | 37.64, 11.33 |
| Year living with HIV (mean, SD) | 15.78, 8.95 | 16.08, 8.90 |
| Male Sex | 103 (65.61) | 98 (66.22) |
| Born in PR | 141 (89.81) | 126 (85.14) |
| Never married | 71 (45.22) | 79 (53.38) |
| Education ≤ 12 years | 104 (66.24) | 89 (60.14) |
| Annual family income < $15,000 | 127 (81.41) | 120 (82.19) |
| Employed | 36 (22.93) | 38 (25.68) |
| Government Health Insurance | 118 (75.16) | 114 (78.08) |
| Homosexual/bisexual sexual orientation | 35 (22.29) | 40 (27.03) |
| Adherent to ART | 92 (59.74) | 81 (54.73) |
| Had heard of CTs before today | 89 (56.69) | 83 (56.08) |
| Previously participated in a non-cancer related CT | 27 (17.76) | 30 (20.83) |
P-value from Chi-square test for categorical variables and t-test or Wilcoxon’s Rank Sum for continuous variables. No significant differences (p>0.05) were observed between study groups.
Table II.
Perception of anal cancer and CTs of study participants at baseline, by study group (n=305)*
| Items** | Control (n=157) Mean, SD | Intervention (n=148) Mean, SD |
|---|---|---|
| I have greater possibilities of developing anal cancer in comparison with other people with my same age | 3.10, 1.72 | 3.30, 1.61 |
| I am very worried about developing anal cancer | 3.85, 1.71 | 4.14, 1.46 |
| Anal cancer is a hopeless disease | 3.03, 1.70 | 2.72, 1.66 |
| Being diagnosed with anal cancer scares me | 4.38, 1.31* | 4.09, 1.50* |
| Participating in CTs to detect anal cancer at earlier stages will help to protect my health | 4.90, 0.53 | 4.95, 0.26 |
| CTs help people living with HIV to fight against cancer | 4.87, 0.53 | 4.86, 0.50 |
P-value from t-test or Wilcoxon’s Rank Sum. Significant differences (p<0.05) were observed between study groups.
A 1 to 5 Likert scale was used to evaluate items related to perceived susceptibility of anal cancer and CTs.
Participant follow-up.
Fifty participants were lost at 1st follow-up and 89 at 2nd follow-up, for an overall retention rate of 71%; retention rates did not vary between the intervention and control arms (p > 0.05) (Figure 2). When comparing people with complete vs. incomplete follow-up, we found that there were no statistically significant differences in socio-demographic and behavioral characteristics between participants (p > 0.05) (data not shown). Thus, we used the entire sample (including people with incomplete follow-up) for all the analyses.
Figure 2.
Consort Flow Diagram
Study outcomes.
Self-perceived awareness of CTs increased in both control and intervention groups from 56.7% and 56.1% at baseline to 81.3% and 80.2% at 1st follow-up (p < 0.001) respectively, although no significant difference between the intervention and control groups was observed (RRadjusted = 1.00, 95% CI: 0.88–1.13). In regard to awareness of HRA, there were a significant increase from 10.8% and 13.5 at baseline to 62.7% and 63.6% at 1st follow-up in the control and intervention groups (p <0.001), respectively, but no significant difference between control and intervention groups was observed (Table III). Meanwhile, although there was an increase in knowledge about CT from 57.9% % and 65.5% at baseline to 66.4% and 71.9% at 1st follow-up in the control and intervention groups, respectively, this results was not statistically significant (p > 0.05); no significant difference between control and intervention groups was observed (RR=1.0, 95% CI: 0.88–1.14). Willingness to participate in a CT that uses HRA at baseline and 1st follow-up were 73.2% and 80.6% in control group, and 71.6% and 84.3% in intervention group. The increase in willingness across time was significant in both groups (p < 0.05 at 1st follow-up). However, there were no significant difference in willingness between the intervention and control groups (RRadjusted =0.97, 95% CI: 0.88–1.08) (Table III). The main reasons reported during the first follow-up for not being interested in participating in a CT that uses HRA among participants who reported not being willing to do so (n=26) were not being interested in participating (26.9%) and being scared (of participating, the procedure, or of its possible side effects) (19.2%). Only one participant reported being concerned of being a “guinea pig” (3.9%). Reasons did not vary between persons in the intervention and control arms (p > 0.05) (data not shown).
Table III.
The effect of the intervention on awareness of CT and HRA, knowledge of CT and willingness to participate in a CT during the first follow-up (n=305).
| Characteristics& | Control | Intervention | RR crude (95%CI)* | RR adjusted (95%CI)** | ||
|---|---|---|---|---|---|---|
| Baseline (n=157) n (%) | 1st follow-up (n=134) n (%) | Baseline (n=148) n (%) | 1st follow-up (n=121) n (%) | |||
| Awareness about CT | 0.99 (0.87, 1.11) p=0.81 | 1.00 (0.88, 1.13) p=0.95 | ||||
| Yes | 89 (56.69) | 109 (81.34) | 83 (56.08) | 97 (80.17) | ||
| No/Don’t know | 68 (43.31) | 25 (18.66) | 65 (43.92) | 24 (19.83) | ||
| Awareness about HRA | 1.02 (0.84, 1.22) p=0.88 | 1.03 (0.84, 1.26) p=0.79 | ||||
| Yes | 17 (10.83) | 84 (62.69) | 20 (13.51) | 77 (63.64) | ||
| No/Don’t know | 140 (89.17) | 50 (37.31) | 128 (86.49) | 44 (36.36) | ||
| Knowledge about CT | 1.08 (0.92, 1.28) p=0.34 | 1.00 (0.88, 1.14) p=0.98 | ||||
| Correct | 91 (57.96) | 89 (66.42) | 97 (65.54) | 87 (71.90) | ||
| Incorrect | 66 (42.04) | 45 (33.58) | 51 (34.46) | 34 (28.10) | ||
|
Willingness to participate in a CT that
uses HRA |
1.05 (0.93, 1.17) p=0.44 | 0.97 (0.88, 1.08) p=0.64 | ||||
| Yes | 115 (73.25) | 108 (80.60) | 106 (71.62) | 102 (84.30) | ||
| No/Don’t know | 42 (26.75) | 26 (19.40) | 42 (28.38) | 19 (15.70) | ||
Risk ratios compare the intervention vs. control groups at the first follow-up (control group used as reference);
Risk ratios adjusted by outcome at baseline, sex, age, and being diagnosed with anal cancer scares me at baseline.
Regarding if participants called to receive orientation about ANCHOR, 14.6% of participants in the control group and 24.8% of those in the intervention group called. In multivariable analysis, participants in the intervention group were 66% more likely of having called ANCHOR to receive orientation about the study than those in the control group (RRadjusted = 1.66, 95% CI: 1.00–2.76, p=0.05). In regard to people who completed ANCHOR’s telephone-based pre-screening evaluation, 10.2% of participants in the control group and 18.2% of participants in the intervention group completed this evaluation. In the crude model, those in the intervention group were 79% more likely (RRcrude=1.79, 95% CI: 1.01–3.18, p=0.05) to complete ANCHOR’s pre-screening evaluation than those in the control group. Similar results were seen in the covariate adjusted analysis, although the association became marginally significant (RR adjusted=1.70, 95% CI: 0.95–3.03; p = 0.07) (Table IV).
Table IV.
The effect of the intervention on participant calls and screening into the ANCHOR study (n=305).
| Characteristics | Control | Intervention | RR crude (95%CI)* | RR adjusted (95%CI)** |
|---|---|---|---|---|
| Called to receive orientation about ANCHOR (n=266)† |
1.70 (1.03, 2.81) p=0.04 | 1.66 (1.00, 2.76) p=0.05 | ||
| Yes | 20 (14.60) | 32 (24.81) | ||
| No | 117 (85.40) | 97 (75.19) | ||
| Completion of pre-screening telephone evaluation to determine eligibility into ANCHOR (n=305) | 1.79 (1.01, 3.18) p=0.05 | 1.70 (0.95, 3.03) p=0.07 | ||
| Yes | 16 (10.19) | 27 (18.24) | ||
| No | 141 (89.81) | 121 (81.76) |
n=266 (87%), as 13% of participants were lost to follow-up in both the 1st and 2nd follow-up surveys; no significant differences (p>0.05) were observed in losses to follow-up of this variable between persons in the intervention and persons in the control group.
Crude risk ratios compare the intervention vs. control groups at 2 weeks (control group used as reference);
Risk ratios adjusted by sex, age, and being diagnosed with anal cancer scares me at baseline.
Among participants who had not called to receive orientation about the ANCHOR study by the first follow-up (n=224), the most common reasons included lack of time/was to busy (30.3%), due to work/study (12.9%), and they were waiting to receive the follow-up call/ did not know that he/she had to call (11.6%) (Table V). Being scared of participating or of side effects was reported by less than 3% of participants and less than 1% mentioned distrust of researchers or concern of being a “guinea pig” as reasons for not having called. Similar results were observed in the intervention (11.2%) and control group (11.9%) with regard to not having called the ANCHOR study because of not knowing that he/she had to initiate the call (p > 0.05). Although a higher proportion of persons in the intervention than in the control arm (10.2% vs. 4.7%) said that they had forgotten to call, this difference was not statistically significant (p = 0.12). None other reason reported differed between persons in the intervention and control arms (p > 0.05).
Table V.
Most common reasons for not having called to receive orientation about the study during the 1st follow-up, among participants who had not called at that time (n= 224)*
| Control (n=126) n (%) | Intervention (n=98) n (%) | Total n (%) | |
|---|---|---|---|
| Lack of time/was busy | 41 (32.54) | 27 (27.55) | 68 (30.36) |
| Work/study | 16 (12.70) | 13 (13.27) | 29 (12.95) |
| I was waiting to receive your call/did not know understand that I had to call | 15 (11.90) | 11 (11.22) | 26 (11.61) |
| Not interested | 13 (10.32) | 5 (5.10) | 18 (8.04) |
| I do not have transportation | 10 (7.94) | 7 (7.14) | 17 (7.59) |
| I forgot to call | 6 (4.76) | 10 (10.20) | 16 (7.14) |
| They did not provide enough information | 7 (5.56) | 3 (3.06) | 10 (4.46) |
| Lost contact information | 2 (1.59) | 5 (5.10) | 7 (3.13) |
| Problems with my phone | 1 (0.79) | 4 (4.08) | 5 (2.23) |
| My doctor has not recommended it | 3 (2.38) | 1 (1.02) | 4 (1.79) |
| I was waiting to talk with the doctor | 2 (1.59) | 2 (2.04) | 4 (1.79) |
| I am scared to participate/scared of possible side effects | 4 (3.17) | 0 (0.00) | 4 (1.79) |
| I am scared about the results | 3 (2.38) | 0 (0.00) | 3 (1.34) |
| I feel healthy | 2 (1.59) | 0 (0.00) | 2 (0.89) |
| Moved from PR | 2 (1.59) | 0 (0.00) | 2 (0.89) |
| Laziness | 0 (0.00) | 2 (2.04) | 2 (0.89) |
| I do not trust the researchers | 1 (0.79) | 0 (0.00) | 1 (0.45) |
| I am concerned of being a “guinea pig” | 1 (0.79) | 0 (0.00) | 1 (0.45) |
Chi-square and Fishers exact test (when cells had counts below 5) used for comparing results in the intervention and control groups. Items with cells with counts of 0 were not compared. No significant differences observed between persons in the intervention and control arms (p>0.05). Participants could report more than one reason.
Discussion
This behavioral RCT evaluates the effect of an educational video in Spanish on increasing participation of Hispanic PLWHA into an anal cancer CT. The video is likely to be more effective than printed materials because it actively (rather than passively as with printed materials) engaged the PLWHA. Furthermore, the content of the video showed people including PLWHA responding to their circumstances and the opportunities presented by the CTs. Thus, the opportunities of active engagement were likely to be perceived to have more positive impact than print materials. Participants in the intervention group had 2-fold higher odds of having called ANCHOR to receive orientation about the study than those in the control group, suggesting the greater effectiveness of the video in combination with printed materials, over just receiving printed materials. Regarding completion of ANCHOR’s pre-screening telephone evaluation, PLWHA in the intervention group were more likely to complete the evaluation than those in the control group, although this result remained only marginally significant in covariate adjusted analysis (p=0.07). Nonetheless, our results support that our educational video was able to produce more calls within this RCT. Video effectiveness may be explained by the fact that written materials are a passive act, while in the video we engage patients in an entertaining medium, which presents messages visually through persons with whom they may identify themselves with. Furthermore, given low literacy of patients with HIV (63% of study participants had ≤ 12 years of education), the video might have been a more effective channel to engage in the topic of anal cancer and CTs. The effectiveness of our video is also consistent to findings from a systematic review (10), that showed that although video’s that present didactic information might increase health literacy, those, such as ours, that use narrative presentation (real people filmed while enacting scenes), are more effective in modifying behavior change. Few studies have evaluated the effect of videos on CT recruitment outcomes that involve behavioral actions, including calls and screening into a trial. A randomized study in lung cancer patients in the US found higher enrollment on therapeutic CTs (17.5 vs. 11.1%) in the group that received the educational video versus the controls, although this difference was not statistically significant (p > 0.05); only a significant difference in patients self-assessed likelihood to enroll on CTs was observed between intervention and control arm (19). Also in the US, a study in African American and White female patients neither showed significant increases in enrollment in breast cancer trials in women after receiving an educational video, versus those who just received standard of care; although the authors recommended the need for the development of culturally targeted videos to have an impact on CT attitudes and enrollment (20). Similarly, a RCT of cancer patients in the United Kingdom did not find an effect of an educational video on CT recruitment, observing similar refusal rates in the intervention and control arms, although the intervention increased knowledge and reduced anxiety about CTs (12). Nonetheless, a cohort study in lung and esophageal cancer patients in the US had very high enrollment rates in the group that received CT lay navigation, that included an educational video (95% of patients offered a CT consented to participate and 76% enrolled) (21).
Main barriers reported by participants for not having called the ANCHOR trial to receive orientation included lack of time/being busy (30%) and because of work/study (13%); however, barriers did not differ between the intervention and control groups. A systematic review also identified time commitment as one important barrier for CT enrollment (22). Other frequently reported barriers for CT participation include mistrust of research and the medical system, perceived harms, transportation, lack of education about CTs and cancer knowledge, and fear resulting from a diagnosis of cancer and fears related to various aspects of CTs (22). Among these, lack of transportation (7.6%) and lack of enough information about the CT (4.5%) were reasons reported within our study, although these were not as common as time and work/study barriers. Meanwhile, although mistrust and fear towards medical research, that are usually attached to historical events where fundamental ethical principles were violated, can negatively influence participation in HIV/AIDS CTs (4,23,24), mistrust of researchers or clinical research was extremely low in our study population (0.45%). Furthermore, not wanting to be a “guinea pig” was reported by only one study participant (0.45%). This is much lower than the report of this barrier in a study of the US AIDS Clinical Trials Group (11% overall and 8–13% for Hispanics)(8). Fear to participate or of possible side effects (1.8%), or of the results (1.3%) were also uncommon in our study. Thus, these results highlight the strength of our population for performing cancer prevention CTs among PLWHA, as willingness is high and patient mistrust is low.
Among other study findings, awareness about the concept of HRA and CTs increased significantly across time. A significant increase was also observed for willingness to participate in a CT that uses HRA, although not for knowledge about CTs in which increases were not significant. Observed increases were similar in both the intervention and control groups and could be explained by the fact that participants in the control arm received printed educational materials, which could have helped them to increase awareness and willingness to participate, similarly to persons in the intervention group. Thus, these results show that printed materials do influence participants, particularly in awareness and willingness of CTs and HRA, although the addition of the video was crucial to cause behavior change (calls and screening within the ANCHOR trial). To our knowledge, no previous study has evaluated the effectiveness of a video exclusively among Hispanics, but other studies have also shown an effect of educational videos in increasing knowledge, and also willingness to participate in CTs, with smaller effects on recruitment (11,19,25). A study in the US that evaluated patients attitudes and preferences about participation in CTs, showed that 68% were interested in participate in a CT, but 82% of them were not aware of readily available information about CTs specific to their illness and expected their treating physician to inform them (26). Thus, this highlights the relevance of developing educational materials, such as our video, that can help educate patients of CT related topics.
Strengths and Limitations.
Among strengths, a RCT design is the gold standard used to evaluate the effect of an intervention on study outcomes. In addition, results are generalizable to PLWHA aged ≥35 years old attending all government-based immunology clinics of PR, given that all clinics participated within the randomization process. Furthermore, recruitment (99%) and follow-up rates where very high in both intervention and control groups, and information of the primary study outcomes surpassed 87% (calls) and 100% (completion of screening survey). Also, there were no differences in participants with complete follow-up information and those who were lost to follow-up with respect to socio-demographic and behavioral characteristics, reducing the potential for bias. Although our recruitment rate is based only on participants who were screened for eligibility into the study, recruitment was high considering values reported in other interventions aiming to increase enrollment for cancer (17.5%) (19) and HIV CTs (49.3%) (27).
A systematic review in UK that evaluated recruitment rates in CTs from 2002 to 2008 found that out of 73 studies, only 55% had a successful recruitment, with only 23% of them achieving a rate between 80% but less than 100% of their target (28). In addition, clinic randomization minimized contamination of the groups. Among limitations, due to the timing of the study and delays in patient recruitment within the clinic in PR, recruitment and retention of participants into the ANCHOR trial could not be evaluated within the study, nonetheless, calls and telephone-based pre-screening in the trial were evaluated, as proxies of recruitment. Also, the power of our study was limited (<80%) with respect to the primary study outcomes. In fact, a post-hoc power estimation was calculated for CT recruitment outcomes. For the outcome of called to received orientation about ANCHOR, the power of our study was 67%, while for the outcome of completion of pre-screening telephone evaluation to determine eligibility to the ANCHOR study, the power of the study was 59%. Finally, the effect of the intervention could be underestimated, as close to 12% of participants reported that they did not call the ANCHOR trial, as it was not clear to them that they had to initiate the call.
Conclusions
This is the first study to evaluate the effect of an educational video in increasing calls and screening outcomes within an anal cancer CT, and the first using Spanish language and Hispanic speakers in the US; with study results being relevant to PLWHA. Although printed materials showed to be effective and resulted in similar increases in CTs and HRA awareness, as well as willingness, among adults in the intervention and control arms, the video seemed more effective in causing behavior change among participants, making them more likely to call and get screened in the ANCHOR trial. Although results for this outcome were marginally significant, this intervention could be used for future recruitment and retention plans within ANCHOR and other anal cancer CTs.
Future research should further explore reasons for apparent efficacy of this educational video, and should assess its efficacy in other PLWHA populations and in other languages besides Spanish. Focus groups could be done to expand and address barriers to for having called to ANCHOR trial in this population, to better understand how we could overcome these barriers, and what solutions may work. Future research could also explore additional strategies to boost the efficacy of the intervention, including making sure that participants understand that they should call the trial for recruitment if they are interested in participating on it, or changing the strategy so that the trial’s coordinator calls them to determine if they are interested in participating on it. Also, it is important to highlight that many participants had the Obama Phone program (also known as Lifeline Assistance), which assigns a limited number of minutes per month for phone use. This limited our ability to contact participants for follow-up interviews if the minutes for that month were already used, and also their interest/ability to use the phone for study participation, as this would imply spending their available minutes. Thus, given that the most common barrier to participant follow-up was phone contact and access, a future incentive could consider giving participants access to phone cards with additional minutes, instead of giving an economic incentive in cash dollars. Also, it will be important to acknowledge experiences with CTs within specific subgroups of HIV-infected persons (i.e. men who have sex with men, intravenous drug users, women), as well as cultural differences in the settings this video is intended to be implemented, as experiences and intentions might vary. Furthermore, the use of educational videos on other cancer prevention topics should be explored in these and other minority groups.
Funding:
This study was funded by the UPRCC/MDACC Partnership for Excellence in Cancer Research (Award Number U54 CA096297/CA096300); and partially supported by the University of California Davis Comprehensive Cancer Center (Award Number U54 CA153499/ CA093373), through funding of the National Cancer Institute, and by the Puerto Rico Clinical and Translational Research Consortium (Award Number U54MD007587), through funding of the National Institute of Minority Health and Health Disparities, of the National Institutes of Health.
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References:
- 1.Colón-López V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, et al. Anal Cancer Risk Among People With HIV Infection in the United States. J Clin Oncol [Internet]. 2017. Nov 15 [cited 2017 Dec 20];JCO.2017.74.929. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29140774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hillman RJ, Cuming T, Darragh T, Nathan M, Berry-Lawthorn M, Goldstone S, et al. 2016 IANS International Guidelines for Practice Standards in the Detection of Anal Cancer Precursors. J Low Genit Tract Dis [Internet]. 2016. Oct [cited 2017 Aug 25];20(4):283–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27561134 [DOI] [PubMed] [Google Scholar]
- 3.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract [Internet]. 2013. Nov [cited 2017 Aug 25];9(6):267–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24130252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King TE. Racial Disparities in Clinical Trials. N Engl J Med [Internet]. 2002. May 2 [cited 2017 Dec 2];346(18):1400–2. Available from: http://www.nejm.org/doi/abs/10.1056/NEJM200205023461812 [DOI] [PubMed] [Google Scholar]
- 5.Murthy VH, Krumholz HM, Gross CP. Participation in Cancer Clinical Trials. JAMA [Internet]. 2004. Jun 9 [cited 2017 Aug 25];291(22):2720. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15187053 [DOI] [PubMed] [Google Scholar]
- 6.Byrne MM, Tannenbaum SL, Glück S, Hurley J, Antoni M. Participation in Cancer Clinical Trials. Med Decis Mak [Internet]. 2014. Jan 29 [cited 2017 Aug 25];34(1):116–26. Available from: http://journals.sagepub.com/doi/10.1177/0272989X13497264 [DOI] [PubMed] [Google Scholar]
- 7.Bernard-Davila B, Aycinena AC, Richardson J, Gaffney AO, Koch P, Contento I, et al. Barriers and facilitators to recruitment to a culturally-based dietary intervention among urban Hispanic breast cancer survivors. J racial Ethn Heal disparities [Internet]. 2015. Jun [cited 2017 Aug 25];2(2):244–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26557471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heumann C, Cohn SE, Krishnan S, Castillo-Mancilla JR, Cespedes M, Floris-Moore M, et al. Regional variation in HIV clinical trials participation in the United States. South Med J [Internet]. 2015. Feb [cited 2017 Aug 25];108(2):107–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25688896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu Abed M, Himmel W, Vormfelde S, Koschack J. Video-assisted patient education to modify behavior: A systematic review. Patient Education and Counseling. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Banda DR, Mete M, Swain SM. A clinical trial with culturally appropriate video to increase participation of African Americans in cancer clinical trials. J Clin Oncol [Internet]. 2011. Sep 20 [cited 2017 Aug 25];29(27_suppl):159–159. Available from: http://ascopubs.org/doi/10.1200/jco.2011.29.27_suppl.159 [Google Scholar]
- 12.Hutchison C, Cowan C, McMahon T, Paul J. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. Br J Cancer [Internet]. 2007. Sep 17 [cited 2017 Aug 25];97(6):705–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17848908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuong W, Larsen ER, Armstrong AW. Videos to influence: a systematic review of effectiveness of video-based education in modifying health behaviors. J Behav Med [Internet]. 2014. Apr 28 [cited 2017 Aug 25];37(2):218–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23188480 [DOI] [PubMed] [Google Scholar]
- 14.Calderon Y, Cowan E, Nickerson J, Mathew S, Fettig J, Rosenberg M, et al. Educational Effectiveness of an HIV Pretest Video for Adolescents: A Randomized Controlled Trial. Pediatrics. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall EW, Sanchez TH, Stein AD, Stephenson R, Zlotorzynska M, Sineath RC, et al. Use of videos improves informed consent comprehension in web-based surveys among internet-using men who have sex with men: A randomized controlled trial. J Med Internet Res. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid JS, McGraw CA, Minor BL, Conde JG, Pawluk R, Lin M, et al. Procurement of shared data instruments for Research Electronic Data Capture (REDCap). J Biomed Inform. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook DA, Artino AR. Motivation to learn: an overview of contemporary theories the cross-cutting edge. Med Educ Med Educ Educ [Internet]. 2016. [cited 2018 Jun 20];50(50):997–1014. Available from: www.mededuc.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaskerud JH. CULTURAL COMPETENCE: WHAT IS IT? Issues Ment Health Nurs [Internet]. 2007. Jan 9 [cited 2018 Jun 21];28(1):121–3. Available from: http://www.tandfonline.com/doi/full/10.1080/01612840600998154 [DOI] [PubMed] [Google Scholar]
- 19.Du W, Mood D, Gadgeel S, Simon MS. An Educational Video to Increase Clinical Trials Enrollment among Lung Cancer Patients. J Thorac Oncol [Internet]. 2008. Jan [cited 2017 Aug 25];3(1):23–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1556086415311989 [DOI] [PubMed] [Google Scholar]
- 20.Du W, Mood D, Gadgeel S, Simon MS. An educational video to increase clinical trials enrollment among breast cancer patients. Breast Cancer Res Treat [Internet]. 2009. Sep [cited 2017 Aug 25];117(2):339–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19152024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cartmell KB, Bonilha HS, Matson T, Bryant DC, Zapka JG, Bentz TA, et al. Patient participation in cancer clinical trials: A pilot test of lay navigation. Contemp Clin trials Commun [Internet]. 2016. Aug 15 [cited 2017 Aug 25];3:86–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27822566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer [Internet]. 2008. Jan 15 [cited 2017 Aug 25];112(2):228–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18008363 [DOI] [PubMed] [Google Scholar]
- 23.Castillo-Mancilla JR, Cohn SE, Krishnan S, Cespedes M, Floris-Moore M, Schulte G, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials [Internet]. 2014. [cited 2017 Aug 25];15(1):14–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24518211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks RA, Newman PA, Duan N, Ortiz DJ. HIV vaccine trial preparedness among Spanish-speaking Latinos in the US. AIDS Care [Internet]. 2007. Jan 10 [cited 2017 Aug 25];19(1):52–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17129857 [DOI] [PubMed] [Google Scholar]
- 25.Geller MA, Downs LS, Judson PL, Ghebre R, Argenta PA, Carson LF, et al. Learning about ovarian cancer at the time of diagnosis: video versus usual care. Gynecol Oncol [Internet]. 2010. Nov [cited 2017 Aug 25];119(2):370–5. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0090825810005202 [DOI] [PubMed] [Google Scholar]
- 26.Sood A, Prasad K, Chhatwani L, Shinozaki E, Cha SS, Loehrer LL, et al. Patients’ attitudes and preferences about participation and recruitment strategies in clinical trials. Mayo Clin Proc [Internet]. 2009. Mar [cited 2017 Aug 25];84(3):243–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19252111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwadz M, Cleland CM, Belkin M, Ritchie A, Leonard N, Riedel M, et al. ACT2 peer-driven intervention increases enrollment into HIV/AIDS medical studies among African Americans/Blacks and Hispanics: A cluster randomized controlled trial. AIDS Behav [Internet]. 2014. Dec [cited 2017 Dec 21];18(12):2409–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24961193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sully BGO, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials [Internet]. 2013. Jun 9 [cited 2017 Aug 27];14:166. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23758961 [DOI] [PMC free article] [PubMed] [Google Scholar]