Figure 5.
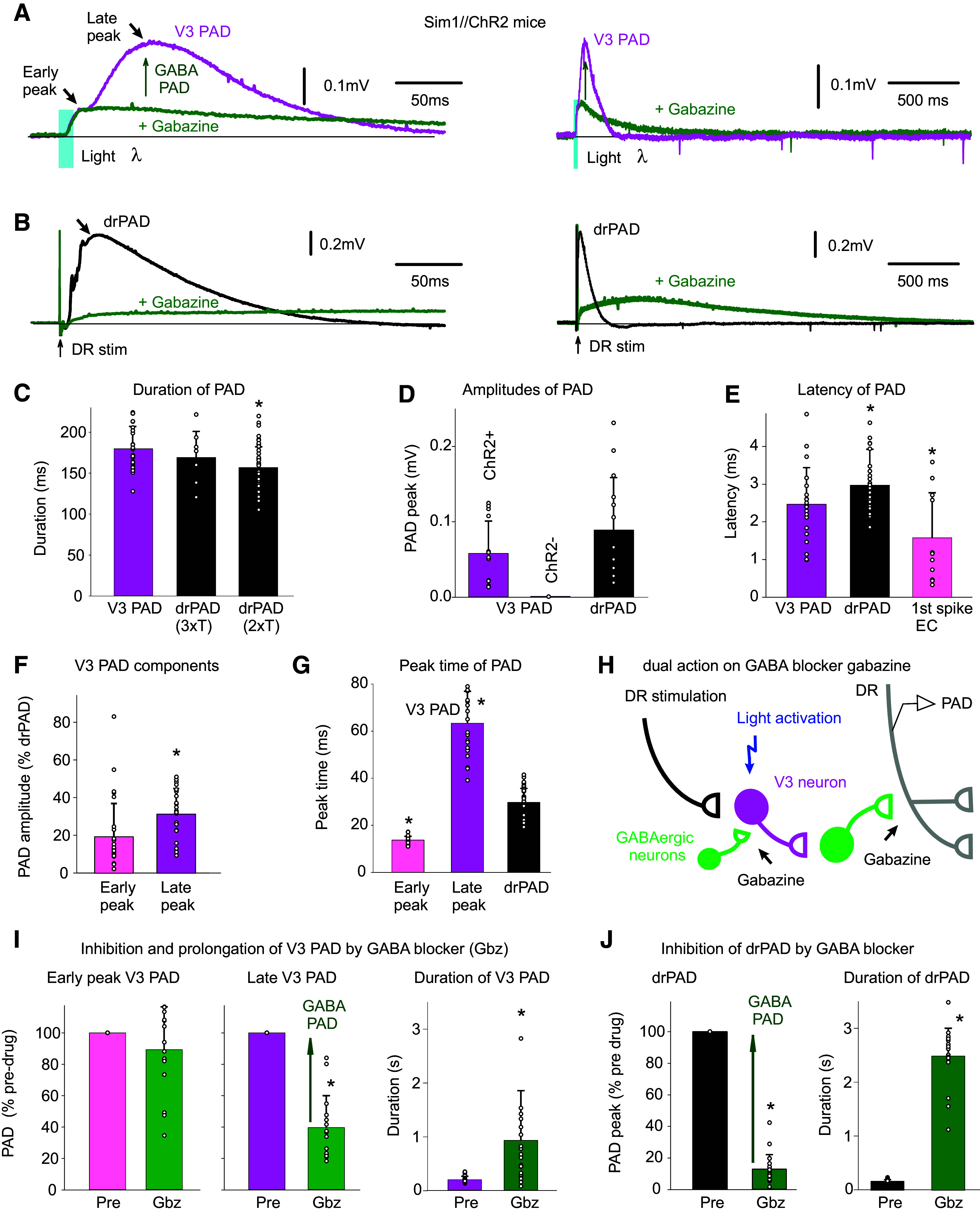
V3 neurons evoke a PAD (V3 PAD), partly mediated by GABAA receptors. A: brief optogenetic activation of V3 neurons in Sim1//ChR2 mice (10 ms pulse, λ = 447 nm laser, 0.7 mW/mm2, 3xT, columnated laser light aligned axially to maximally activate V3 neurons over multiple segments, as in Fig. 4B) evokes a PAD (termed V3 PAD) recorded in DR (S3 DR, DRP recorded with grease-gap method), with an early peak and a late peak, as indicated. Blocking GABAA receptors with gabazine (50 µM) blocks the late V3 PAD, but reveals a very long-lasting PAD that starts at the early peak, as shown on the expanded time scale on the right. B: similar to A, but brief DR stimulation evokes a drPAD that is mostly blocked by gabazine, but again reveals a very long-lasting PAD. C: group averages of durations of V3 PAD and drPAD evoked by 2xT and 3xT DR stimulation, before gabazine. *drPAD significantly less than V3 PAD, n = 28 V3 PADs and 50 drPADs from 6 and 9 mice, respectively, P < 0.05. D: group averages of maximum PAD amplitudes (late peak for V3 PAD), in Sim1//ChR2+ (same mice) and Sim1ChR2- mice (n = 12 from 4 mice), *drPAD significantly larger, P < 0.05. E: central latency of V3 PAD, drPAD, and first spike evoked in V3 neurons (the latter as in Fig. 4G. n = 11 in 6 mice), *significantly different than V3 PAD latency, P < 0.05. F and G: amplitude and peak time of the early and late peaks in V3 PAD, *late peak amplitude significantly larger than early peak, or V3 peak times significantly different than drPAD peak time, same mice, P < 0.05. H: schematic of trisynaptic circuit underlying PAD with GABAergic inhibition of V3 neurons, explaining disinhibition of late PAD with gabazine. I and J: changes in V3 PAD and drPAD with gabazine, *significant change in V3 PAD (n = 16, from 6 mice) and drPAD (n = 21 from 5 mice) amplitude and duration, P < 0.05. DR, dorsal root; PAD, primary afferent depolarization.
