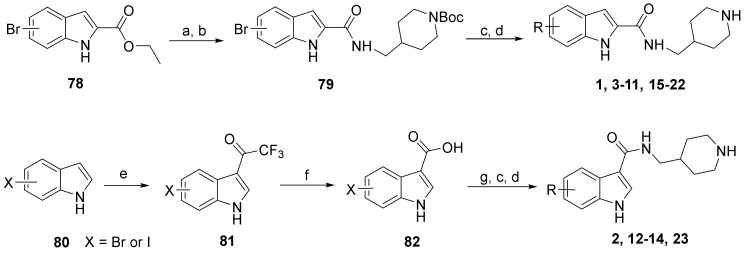
Scheme 1.
Synthesis of compounds 1–23. Reagents and conditions: (a) NaOH, THF-H2O, 50 °C, 5 h; (b) 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate, N,N-diisopropylethylamine, DMF, 4-aminomethyl-1-Boc-piperidine, 12 h; (c) aryl boronic acid or aryl 4,4,5,5-tetramethyl-1,3,2-dioxaborolane, tetrakis(triphenylphosphine)palladium, Na2CO3, 1,4-dioxane-H2O, 100 °C; (d) HCl (4 N in 1,4-dioxane), CH2Cl2, 0 °C; (e) trifluoroacetic anhydride, 0 °C to room temperature, 2 h; (f) NaOH (20% aq.), 80 °C, 2 days; (g) 4-aminomethyl-1-Boc-piperidine, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide, 1-hydroxybenzotriazole, triethylamine, CH2Cl2.