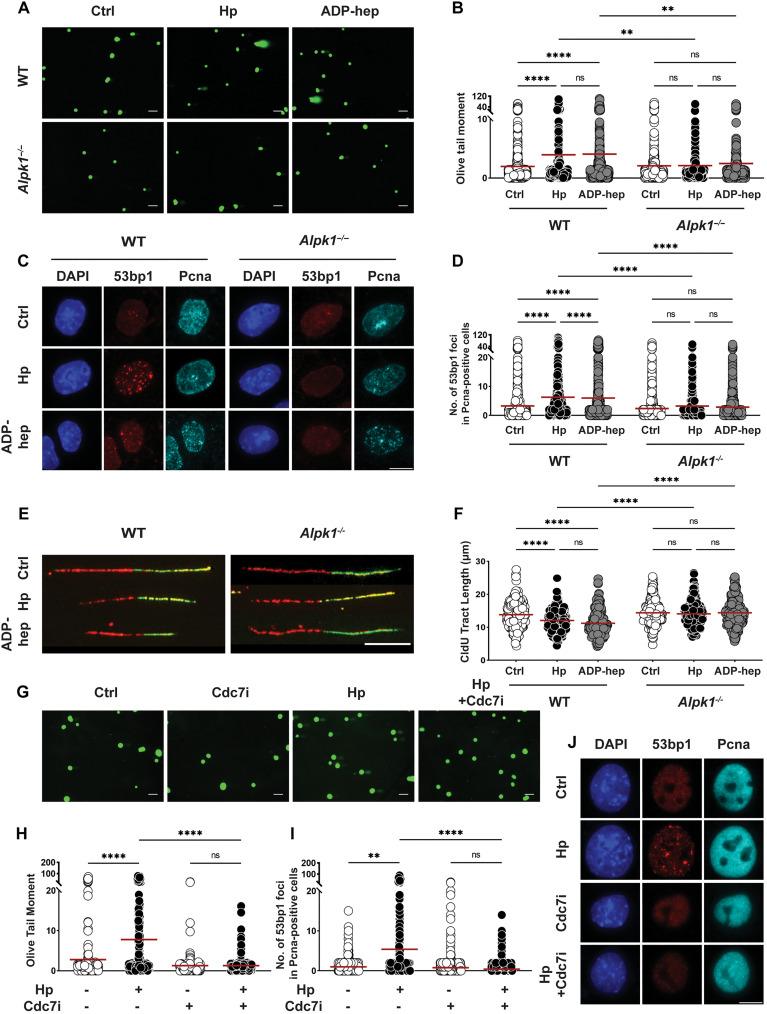
Fig. 2. H. pylori–induced DNA damage and replication stress is dependent on Alpk1 signaling and unimpaired progression through S phase.
(A to F) Antrum-derived organoid cells seeded in 2D were infected with H. pylori strain G27 (MOI of 50) for 6 hours. Cells were harvested and analyzed for the appearance of DNA damage by alkaline comet assay [(A) and (B)] and by 53bp1/Pcna-specific immunofluorescence microscopy [(C) and (D)]; replication stress was quantified by DNA fiber assay [(E) and (F)]. Representative images of comets, 53bp1 foci formation, and DNA fibers are shown in (A), (C), and (E) (scale bars, 10 μm); pooled data from three independent experiments are shown in (B), (D), and (F) [~300 cells are shown per condition in (B), ~1500 cells per condition in (D), and 300 fibers per condition in (F)]. (G to J) Antrum-derived organoid cells seeded in 2D were infected with H. pylori strain G27 (MOI of 50) for 6 hours, in the presence or absence of the Cdc7 inhibitor XL413 at 0.2 μM final concentration. Cells were harvested and analyzed by alkaline comet assay [(G) and (H)] and by 53bp1/Pcna-specific immunofluorescence microscopy [(I) and (J)]. Data are from one experiment and representative of two independently conducted ones. A total of ~150 cells and ~1000 cells per condition are shown in (H) and (I), respectively. Horizontal lines represent means. P values were calculated using one-way analysis of variance (ANOVA); **P < 0.01; ****P < 0.001.