Abstract
BACKGROUND
Superficial temporal artery–middle cerebral artery (STA-MCA) bypass surgery is performed to prevent ischemia and hemorrhage in patients with moyamoya disease. Only a few reports have described aneurysms appearing around the anastomosis site after bypass surgery, and the underlying mechanism remains unknown.
OBSERVATIONS
The present case involved a 62-year-old woman who underwent STA-MCA bypass surgery for ischemic quasi-moyamoya disease at 46 years of age. Postoperatively, she underwent annual magnetic resonance imaging examinations. At 11 years after STA-MCA bypass surgery, a 3-mm aneurysm appeared at the anastomosis site. Four years later, headache developed and the aneurysm had grown to 5 mm. Craniotomy clipping was performed to prevent rupture. The patient was discharged home 2 weeks after surgery without any apparent complications.
LESSONS
Long-term observation is crucial after direct bypass surgery for moyamoya disease. Measures to prevent rupture should be considered for cases involving aneurysm complications.
Keywords: moyamoya disease, anastomosis, aneurysm
ABBREVIATIONS: MCA = middle cerebral artery, STA = superficial temporal artery, STA-MCA = superficial temporal artery–middle cerebral artery
Superficial temporal artery–middle cerebral artery (STA-MCA) bypass surgery is performed to prevent ischemia and hemorrhage in patients with moyamoya disease.1,2 There are few reports of aneurysms appearing at the anastomosis site after STA-MCA bypass surgery for stenosis or occlusion of the main trunk artery. However, aneurysms around the anastomosis site rarely appear after bypass surgery for moyamoya disease. Half of the reported cases involved rupture and had a poor prognosis; therefore, prophylactic treatment is critical.3,4
Illustrative Case
We present the case of a 62-year-old woman with a history of hypertension who presented with lalopathy and left hemiparesis at 46 years of age. She underwent clipping of an aneurysm at the anastomosis site 16 years after undergoing STA-MCA bypass surgery for moyamoya disease. She had a right parietal lobe infarction. Additionally, cerebral angiography showed moyamoya vessels. The beginning of the right middle cerebral artery (MCA) was not visible on angiography. These results indicated the diagnosis of moyamoya disease. The patient underwent STA-MCA bypass surgery to prevent recurrent cerebral infarctions. Her headache disappeared immediately after surgery. At 14 days after surgery, she was discharged home. The patient underwent annual follow-up, including blood pressure control and magnetic resonance imaging. At 11 years after surgery, a 3-mm saccular aneurysm was found at the anastomosis site. Four years later, headache occurred and magnetic resonance imaging showed that the aneurysm had increased to 5 mm. The patient underwent surgery to prevent aneurysm rupture (Fig. 1). Cerebral angiography revealed that the aneurysm was located proximal to the anastomosis in the MCA, with the dome facing the direction of the superficial temporal artery (STA) anastomosis. Furthermore, a 1.7-mm aneurysm was observed adjacent to the aneurysm in the MCA. Right internal carotid angiography showed that the leptomeningeal anastomosis had developed from the anterior cerebral artery and perfused the circumflex area. The aneurysm was assumed to be located directly below the site of the previous craniotomy (Fig. 2). Blood flow from the STA was good; however, perfusion to the right cerebral hemisphere from the MCA was poor. Therefore, we determined that blood flow from the STA should be preserved and decided to perform craniotomy clipping to enable re-anastomosis if the blood vessel is occluded during surgery. Based on the previous craniotomy results, an osteotomy was performed to preserve the bony margin where the STA entered the cranium. A C-shaped incision was made through the dura mater. When we flipped the dura mater, we found that the aneurysm was invading the frontal lobe. The aneurysm was carefully dissected because there were adhesions between the aneurysm and dura mater. The 5-mm aneurysm was clipped, and the 1.7-mm aneurysm was coated. Motor evoked potentials were measured frequently from the time of dural rotation and did not decrease. Postoperative cerebral angiography showed that the aneurysm had disappeared, and diffusion-weighted imaging revealed no ischemia. The patient was discharged home without headache recurrence (Fig. 3).
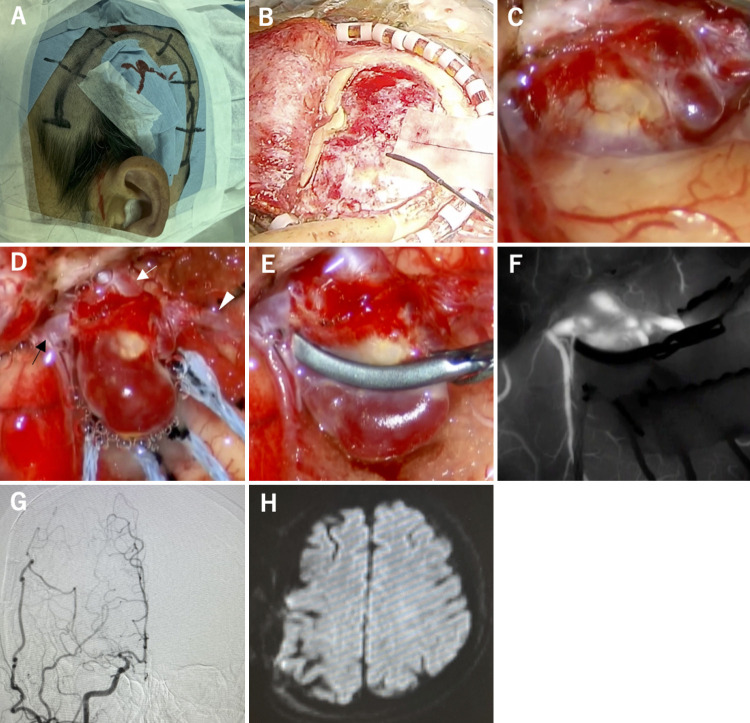
FIG. 1.
Superficial temporal artery–middle cerebral artery (STA-MCA) bypass surgery 16 years previously (A and B). Postoperative course (C and D). A: Cerebral angiography showing stenosis at the origin of the right internal carotid artery and moyamoya vessels. B: STA-MCA end-to-side anastomosis. C: Magnetic resonance angiography at 11 years postoperatively revealing a 3-mm aneurysm at the anastomosis site. D: Magnetic resonance angiography showing that the aneurysm enlarged to 5 mm at the anastomosis site 16 years after surgery.
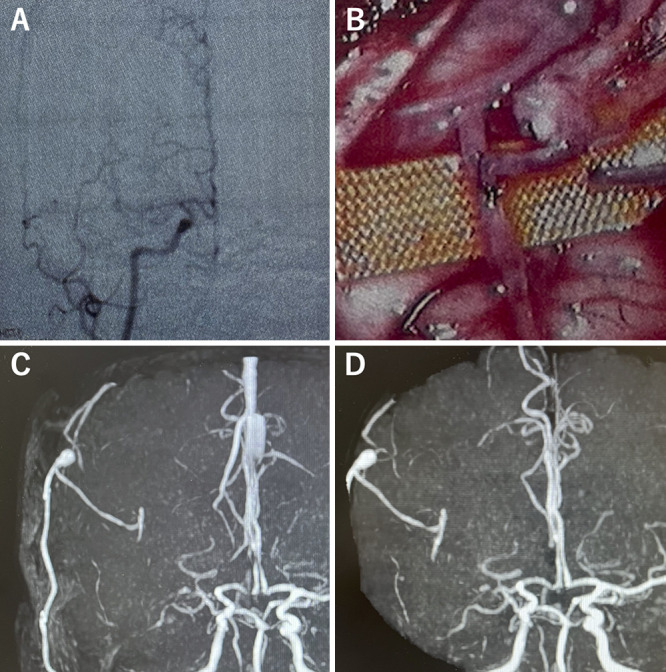
FIG. 2.
Preoperative evaluation. A: Right external carotid arteriography showing good perfusion from the STA to the cerebral isthmus. B: Right internal carotid angiogram showing that the leptomeningeal anastomosis developed from the anterior cerebral artery (ACA). C: Three-dimensional angiography shows that the 5-mm aneurysm is located proximal to the anastomosis in the MCA (thick white arrow). A 1.7-mm aneurysm is noted on the distal MCA (yellow arrowhead). The STA (white arrowhead) is observed proximal to the MCA (thin white arrow) and distal to the MCA (thick yellow arrow). D: Three-dimensional computed tomography angiography shows the aneurysm directly below the site of the previous craniotomy.
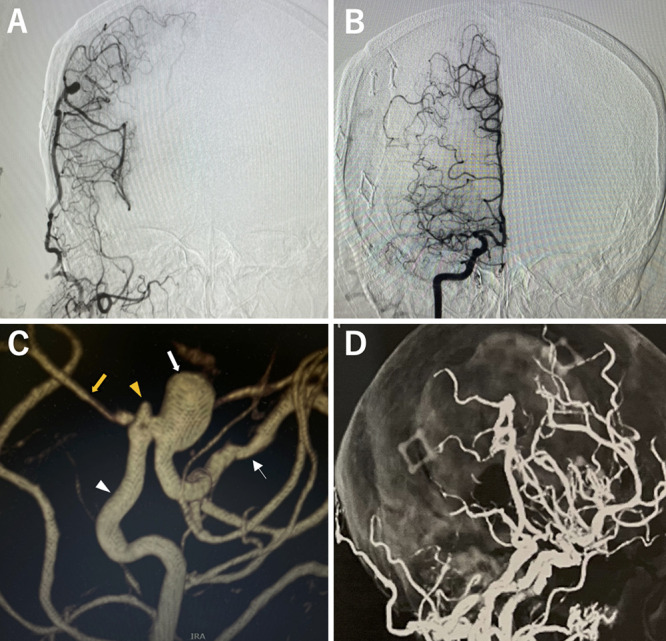
FIG. 3.
Craniotomy clipping. A: The area is enclosed with C-shaped craniotomy. B: Some bone is left at the point of STA inflow after the previous craniotomy is observed. C: The aneurysm is toward the frontal lobe, and the dome is covered by the frontal lobe. D: A saccular aneurysm in the middle cerebral artery wall contralateral to the STA anastomosis. The STA (white arrow) is observed proximal to the MCA (black arrow) and distal to the MCA (white arrowhead). E: Neck clipping. F: Indocyanine green staining shows loss of blood flow in the aneurysm and residual blood flow in the MCA. G: Postoperative internal carotid arteriography shows that the aneurysm disappeared. H: Postoperative diffusion-weighted imaging shows no new ischemia.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
The incidence of intracranial aneurysms with moyamoya disease is estimated to be 3%–14% for adults; furthermore, intracranial aneurysms tend to occur in the main trunk of the circle of Willis and peripheral vessels, such as moyamoya vessels and collateral arteries.5 To the best of our knowledge, there have 4 four cases of postoperative aneurysm complications after STA-MCA bypass surgery for moyamoya disease (Table 1). Two patients developed hemorrhage,3,4,6,7 and 2 incidentally found cases were asymptomatic. The time from postoperative surgery to aneurysm appearance in the 4 reported cases ranged from 6 months to 20 years (mean, 7.7 years). One case involved an aneurysm that grew from 2 mm to 6 mm over a period of 8 years after it first appeared. Because of the relatively young age of patients with moyamoya disease, the postoperative clinical course is long; therefore, the follow-up period is crucial. All 4 patients underwent craniotomy. Although vascular treatment is an option because of recent advances in catheter technology, craniotomy is considered superior because of the possibility of repeat anastomosis. One of the risks associated with craniotomy is the development of a leptomeningeal anastomosis from the dura mater, which may be difficult to dissect. The dura was flipped to the neck of the aneurysm, and the STA and proximal MCA were visible. No new cases of postoperative ischemia were observed. Minimizing dural rotation allows safe treatment, even with craniotomy. Millesi et al. reviewed 17 cases in which aneurysms appeared at the anastomosis site after extracranial-to-intracranial bypass for atherosclerotic changes and found that 10 patients experienced bleeding during the study period.8 In 9 patients, aneurysm formation was associated with progressive headache episodes. In our case, the aneurysm remained unchanged for 3 years after it first appeared; however, 4 years later, headache occurred and an enlarged aneurysm was observed. Intraoperative findings indicated that the enlarged aneurysm was near the frontal lobe; therefore, the headache may have been caused by the mass effect. Furthermore, headache may be a sign of aneurysm enlargement.8 Additionally, Millesi et al.8 have reported that the time to detection of postoperative aneurysms ranged from 2 weeks to 27 years during both the early and late stages. Aneurysms associated with moyamoya disease develop late compared to atherosclerotic lesions, which also develop during the early postoperative period. Regarding the mechanism of the appearance of the aneurysm, based on histopathological examination results of both the vessel wall and aneurysm wall specimens of donor grafts, Fein9 hypothesized that aneurysm formation is time dependent. Fleisher et al.10 postulated that the disruption of the internal elastic layer and tunica media of the arterial anastomosis during bypass surgery may result in traumatic aneurysm formation at the suture line. Kohno et al.11 speculated that the artificial bifurcation created by STA-MCA bypass was exposed to excessive jet flow from the enlarged STA, resulting in spindle-like widening at the anastomosis site, followed by aneurysmal dilation caused by increased hemodynamic forces. In the present case, the aneurysm was not located on the suture line at the anastomosis site; instead, it protruded toward the MCA wall, consistent with the direction of STA inflow at the T-bifurcation. This finding is consistent with the hypothesis that the aneurysm was caused by hemodynamic forces. After bypass surgery, the patient’s blood pressure was well controlled at ≤140 mm Hg. However, because the aneurysm could have been caused by a pressure gradient in the arterial wall, we believe it is crucial to strictly control blood pressure and perform periodic imaging examinations to prevent moyamoya disease and avoid overlooking early symptoms, such as headache.
TABLE 1.
Cases of aneurysms associated with STA-MCA bypass for moyamoya disease
| Authors & Year | Age (yrs)/Sex | Initial Treatment | Interval | Clinical Presentation | Aneurysm Location | Aneurysm Size | Operation | Outcome |
|---|---|---|---|---|---|---|---|---|
| Nishimoto et al., 20053 |
52/F |
STA-MCA bypass |
20 yrs |
ICH |
MCA (not suture line) |
5 mm |
Clipping |
Severe disability |
| Eom et al., 20104 |
51/F |
STA-MCA bypass + EDMS |
6 mos |
ICH |
STA (not suture line) |
26 mm |
Aneurysmectomy |
Moderate disability |
| Yokota et al., 20166 |
54/F |
STA-MCA bypass |
7 yrs |
Incidental/enlargement |
STA (not suture line) |
Grew from 0 to 4 mm over 1 yr |
Clipping |
Good recovery |
| Aburakawa et al., 20177 |
53/F |
STA-MCA bypass + EDMS |
11 yrs |
Incidental/enlargement |
MCA (not suture line) |
Grew from 2 to 6 mm over 8 yrs |
Clipping |
Good recovery |
| Present case | 62/F | STA-MCA bypass | 11 yrs | Headache/enlargement | MCA (not suture line) | Grew from 3 to 5 mm over 4 yrs | Clipping | Good recovery |
ICH = intracerebral hemorrhage; EDMS = encephalo-duro-myo-synangiosis.
Lessons
Long-term observation is critical after direct bypass surgery for moyamoya disease. Surgical intervention should be aggressively considered for aneurysmal complications to prevent rupture.
Acknowledgments
We thank Dr. Kawamata and Dr. Arai for useful discussions and their collaboration during the early stages of this work.
Author Contributions
Conception and design: Eguchi. Acquisition of data: Eguchi. Analysis and interpretation of data: Eguchi. Drafting of the article: Eguchi. Critically revising the article: all authors. Reviewed submitted version of the manuscript: Eguchi. Approved the final version of the manuscript on behalf of all authors: Eguchi. Administrative/technical/material support: Eguchi, Kawamata. Study supervision: Kawamata.
References
- 1. Funaki T, Takahashi JC, Houkin K, et al. Effect of choroidal collateral vessels on de novo hemorrhage in moyamoya disease: analysis of nonhemorrhagic hemispheres in the Japan Adult Moyamoya Trial. J Neurosurg. 2019;132(2):408–414. doi: 10.3171/2018.10.JNS181139. [DOI] [PubMed] [Google Scholar]
- 2. Miyamoto S, Yoshimoto T, Hashimoto N, et al. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial. Stroke. 2014;45(5):1415–1421. doi: 10.1161/STROKEAHA.113.004386. [DOI] [PubMed] [Google Scholar]
- 3. Nishimoto T, Yuki K, Sasaki T, Murakami T, Kodama Y, Kurisu K. A ruptured middle cerebral artery aneurysm originating from the site of anastomosis 20 years after extracranial-intracranial bypass for moyamoya disease: case report. Surg Neurol. 2005;64(3):261–265. doi: 10.1016/j.surneu.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 4. Eom KS, Kim DW, Kang SD. Intracerebral hemorrhage caused by rupture of a giant aneurysm complicating superficial temporal artery-middle cerebral artery anastomosis for moyamoya disease. Acta Neurochir (Wien) 2010;152(6):1069–1073. doi: 10.1007/s00701-009-0550-8. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi JC, Miyamoto S. Moyamoya disease: recent progress and outlook. Neurol Med Chir (Tokyo) 2010;50(9):824–832. doi: 10.2176/nmc.50.824. [DOI] [PubMed] [Google Scholar]
- 6. Yokota H, Yokoyama K, Noguchi H. De novo aneurysm associated with superficial temporal artery to middle cerebral artery bypass: report of two cases and review of literature. World Neurosurg. 2016;92:583.e7–583.e12. doi: 10.1016/j.wneu.2016.05.075. [DOI] [PubMed] [Google Scholar]
- 7. Aburakawa D, Fujimura M, Niizuma K, Sakata H, Endo H, Tominaga T. Navigation-guided clipping of a de novo aneurysm associated with superficial temporal artery-middle cerebral artery bypass combined with indirect pial synangiosis in a patient with moyamoya disease. Neurosurg Rev. 2017;40(3):517–521. doi: 10.1007/s10143-017-0866-4. [DOI] [PubMed] [Google Scholar]
- 8. Millesi M, Wang WT, Herta J, Bavinzski G, Knosp E, Gruber A. De novo aneurysm formation at the anastomosis site incidentally detected 2 years after single-barrel STA-MCA bypass surgery: case report and review of the literature. J Neurol Surg A Cent Eur Neurosurg. 2015;76(4):323–327. doi: 10.1055/s-0034-1376189. [DOI] [PubMed] [Google Scholar]
- 9. Fein JM. Bypass induced cerebral aneurysm. Neurol Res. 1985;7(1):46–52. doi: 10.1080/01616412.1985.11739700. [DOI] [PubMed] [Google Scholar]
- 10. Fleischer AS, Faria MA, Jr, Hoffmann JC., Jr Pseudoaneurysm complicating superficial temporal artery--middle cerebral artery bypass. Surg Neurol. 1979;12(4):305–306. [PubMed] [Google Scholar]
- 11. Kohno K, Ueda T, Kadota O, Sakaki S. Subdural hemorrhage caused by de novo aneurysm complicating extracranial-intracranial bypass surgery: case report. Neurosurgery. 1996;38(5):1051–1055. doi: 10.1097/00006123-199605000-00041. [DOI] [PubMed] [Google Scholar]