Abstract
1-O-alkylglycerols are a class of natural existing lipids with broad biological activities. However, their use in food or agricultural fields remains to be investigated, especially for their antimicrobial activity. In this work, three 1-O-alkylglycerols, 1-O-octanylglycerol (C8Gly1), 1-O-dodecylglycerol (C12Gly1), and 1-O-hexadecylglycerol (C16Gly1), were synthesized in the isolated yields of 71.3–89.8 % and characterized by 1H NMR, 13C NMR, FITR, mass spectra, and HPLC-ESLD. The critical micelle concentration (CMC) of 1-O-alkylglycerols was determined to be 1.65 mmol/L (C8Gly1), 0.33 mmol/L (C12Gly1), and 0.23 mmol/L (C16Gly1) using the pyrene method. C12Gly1 and C16Gly1 had similar surface tensions that are lower than C8Gly1. C8Gly1 can form micelles in aqueous solution with excellent nano-dispersed uniformity and stability. Furthermore, C8Gly1 and C12Gly1 not only displayed good antibacterial activity against Staphylococcus aureus, but they also inhibited the growth of Botryosphaeria dothidea, Monilia fructigena, and Phytophthora capsicum at 400 μg/mL. Thus, the C8Gly1 and C12Gly1 can serve as novel antimicrobial agents in food preservation.
Keywords: 1-O-Alkylglycerol, Synthesis, Surface property, Antibacterial activity, Antifungal activity
Graphical abstract
Highlights
-
•
Three 1-O-alkylglycerol were synthesized in high yields and purity.
-
•
The CMC, surface tension, and water dispersion were determined.
-
•
C8Gly1 and C12Gly1 showed good antibacterial activity against S. aureus.
-
•
C8Gly1 and C12Gly1 also displayed potent antifungal activity.
1. Introduction
Many lipids, including fatty acids and their monoglycerides, showed significant antimicrobial activity by the mechanism of interacting with cell membranes [1,2]. The most powerful and commonly used of these lipids is 1-monolaurin (Fig. 1), a medium-chain fatty acid 1-monoglyceride that outperformed lauric acid due to its higher amphiphilic nature [3,4]. Furthermore, the US Food and Drug Administration declared 1-monolaurin as GRAS (generally recognized as safe) [5]. As structural counterparts of 1-monoglycerides, 1-O-alkylglycerols have yet to be studied extensively, although they have the potential to be more effective since ether bonds have better chemical and metabolic stability than esters [6].
Fig. 1.
Chemical structures of 1-monolaurin and 1-dodecylglycerol.
The principal natural 1-O-alkylglycerols, including chimyl, batyl, and selachyl alcohols, were mainly found in shark liver oil [[6], [7], [8]]. Moreover, they are present naturally in hematopoietic (blood-forming) organs such as bone marrow, spleen, and liver, and they may also be found in neutrophils and human and cow milk [9]. The biological effects of 1-O-alkylglycerols have a wide range of applications, including immunological stimulation [10,11], radiation therapy [12], cancer treatment [13,14], and antimicrobial agent [15]. The antimicrobial effects of 1-O-alkylglycerols give them the potential to replace 1-monoglycerides, not only because of their improved water solubility and surface-active properties but also higher chemical stability, which might broaden their applicability in the food industry.
So far, only 1-dodecylglycerol (Fig. 1) has been extensively explored for its antibacterial and antifungal activities in pharmaceutical science, such as suppression of Streptococcus mutans BHT [16], Candida and Cryptococus [17], Streptococcus faecalis ATCC 9790 [18], and shown to be remarkably effective. Surprisingly, there have been few investigations on the antibacterial and antifungal activities of 1-O-alkylglycerols in the food industry as preservatives. As we all know, avoiding microbiological spoilage and extending product shelf life at various stages of the food chain is an external topic in the food industry. Developing natural chemical preservatives that are not restricted by environmental conditions is essential. Moreover, de Paula et al. demonstrated that the strong intermolecular interaction between 1-O-alkylglycerols and xanthan gum results in a complex with a much-reduced CMC that might be employed as a food additive with antimicrobial activity [19]. The nature of 1-O-alkylglycerols implies there is an urgent need to broaden their antimicrobial evaluation and potential applications.
In the present study, high pure 1-O-alkylglycerols, including 1-O-octanylglycerol (C8Gly1), 1-O-dodecylglycerol (C12Gly1), and 1-O-hexadecylglycerol (C16Gly1), were synthesized and characterized. Their surface property, water dispersion, antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), and antifungal activity against four fungi were studied. C8Gly1 and C12Gly1 were found to have excellent antibacterial and antifungal activities that can be applied as antimicrobial agents in food and agriculture.
2. Materials and experiments
2.1. Materials
Solketal (98 %) was purchased from Sigma-Aldrich (Shanghai, China). Chimyl alcohol (>98 %), 1-bromooctane (99 %), 1-bromododecane (97 %), 1-bromohexadecane (97 %), tetrabutylammonium bromide (99 %), and pyrene (99 %) were all purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The bacteria strains of E. coli (BNCC 336902) and S. aureus (BNCC 186335) were purchased from Bena Culture Collection (Beijing, China). All fungal strains were given by the College of Chemistry of Central China Normal University (Wuhan, China). Other reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Milli-Q water (Millipore, Bedford, MA) was used throughout the study.
2.2. Synthesis of 1-O-alkylglycerols
1-O-alkylglycerols were synthesized by two-step reactions (Fig. 2). 1-bromooctane, 1-bromododecane and 1-bromohexadecane were used to prepare 1-O-octanylglycerol (C8Gly1), 1-O-dodecylglycerol (C12Gly1), and 1-O-hexadecylglycerol (C16Gly1) according to the previously published procedure by Halldorsson et al. [20]. CiGlyi was used for the nomenclature of 1-O-alkylglycerols, where Ci represents the alkyl chain length and Glyi is the number of glycerol moiety linked to the alkyl chain. Characterizations of 1H NMR, 13C NMR, FTIR, and MS are as follows.
Fig. 2.
Synthetic route for 1-O-alkylglycerols using two-step reactions.
C8Gly1 (C11H24O3, molecular weight: 204.17 g/mol). Colorless liquid. 1H NMR (400 MHz, DMSO‑d6) δ 0.85 (t, J = 8.0 Hz, 3H, CH3), 1.25 (s, 10H, CH2), 1.47 (t, J = 8.0 Hz, 2H, CH2CH2O-glycerol), 3.33–3.41 (m, 4H, CH2-glycerol and CH2O-glycerol), 3.56–3.60 (m, 1H, CH-glycerol), 3.95–3.97 (m, 1H, CH-glycerol), 4.12–4.18 (m, 1H, CH-glycerol); 13C NMR (100 MHz, DMSO‑d6) δ 14.1, 22.2, 25.7, 28.8, 28.9, 29.2, 31.4, 66.1, 70.8, 71.3, 74.4; FTIR (KBr, cm−1) ν 3403, 2916, 2848, 1467, 1116, 1045; ESI-MS: m/z 186.22 [M − H2O]+.
C12Gly1 (C15H32O3, molecular weight: 260.23 g/mol). White solid, m.p. 32–34 °C. 1H NMR (400 MHz, DMSO‑d6) δ 0.85 (t, J = 8.0 Hz, 3H, CH3), 1.25 (s, 18H, CH2), 1.46 (t, J = 8.0 Hz, 2H, CH2, CH2CH2O-glycerol), 3.21–3.41 (m, 4H, CH2, CH2-glycerol and CH2O-glycerol), 3.52–3.60 (m, 1H, CH-glycerol), 4.44 (t, J = 8.0 Hz, 1H, CH-glycerol), 4.58 (d, J = 4.0 Hz, 1H, CH-glycerol); 13C NMR (100 MHz, DMSO‑d6) δ 14.0, 22.2, 25.8, 28.9, 29.2, 29.2, 29.4, 31.5, 63.3, 70.6, 72.4; FTIR (KBr, cm−1) ν 3413, 2916, 2848, 1467, 1116, 1047; ESI-MS: m/z 283.36 [M+Na]+.
C16Gly1 (C19H40O3, molecular weight: 316.29 g/mol). White solid, m.p. 63–65 °C (literature 64–66 °C). 1H NMR (400 MHz, DMSO‑d6) δ 0.85 (t, J = 8.0 Hz, 3H, CH3), 1.23 (s, 26H, CH2), 1.43–1.48 (m, 2H, CH2, CH2CH2O-glycerol), 3.21–3.36 (m, 4H, CH2, CH2-glycerol and CH2O-glycerol), 3.52–3.56 (m, 1H, CH-glycerol), 4.45 (t, J = 8.0 Hz, 1H, CH-glycerol), 4.58 (d, J = 4.0 Hz, 1H, CH-glycerol); 13C NMR (100 MHz, DMSO‑d6) δ 14.1, 22.2, 25.8, 28.8, 29.0, 29.2, 29.4, 31.4, 63.3, 70.6, 72.4; FTIR (KBr, cm−1) ν 3364, 2916, 2848, 1474, 1119, 1060; ESI-MS: m/z 339.34 [M+Na]+.
2.3. HPLC-ELSD characterization
An Agilent 1260 HPLC system (Agilent Corporation, Milford, MA, USA) and an Alltech 6000 ELSD detector (Alltech Associates, Inc., Deerfield, IL, USA) were used for the HPLC-ELSD characterization. The chromatography separation was performed on an Inertsil OSD-3 C18 column (4.6 × 150 mm, 5 μm). Methanol (100 %) was used as the mobile phase at a 1.0 mL/min flow rate and a column temperature of 25 °C. The detection condition of ELSD was set as: drift tube temperature of 60 °C, nebulizer gas (N2) flow rate of 2.6 L/min. The injection volume was 20 μL.
2.4. Determination of critical micelle concentration (CMC)
The CMC values of C8Gly1, C12Gly1, and C16Gly1 were measured using a fluorescence spectrophotometer (Hitach, Japan, F4600). The fluorescence spectrum conditions of pyrene aqueous solution were set at a temperature of 26 °C, and the fluorescence spectra were recorded at the wavelength from 350 to 450 nm with excitation slit widths fixed at 5.0 nm and emission slit widths fixed at 2.5 nm. The scan time was fixed at 1200 nm/min, and the wavelength of excitation was 335 nm.
2.5. Measurement of dynamic surface tension
The pendant drop method measured the air-liquid dynamic surface tension of individual samples at the concentration of CMC using a DSA30 automatic tensiometer (Kruss, Germany) at 25 °C. The pendant drop shape was analyzed via Kruss Advance software. Data collection frequency was every second, and all tests were performed for 30 min.
2.6. Determination of Z-average mean diameter, polydispersity index (PDI), and Zeta-potential
Z-average mean diameter, PDI, and Zeta-potential measurements of 1-O-alkylglycerols in aqueous solution at their CMC were performed using a Malvern Zetasizer Nano ZS90 particle analyzer (Malvern Instruments Ltd., Worcestershire, UK). All samples were determined directly without dilution.
2.7. Antibacterial activity
The antibacterial activity of 1-O-alkylglycerols against S. aureus and E. coli were determined in vitro by the 96-well microtiter plate technique. Briefly, 100 μL of bacteria culture (ca. 106 CFU/mL bacterial) and 100 μL of 1-O-alkylglycerol solution were added to wells of microtiter plates. The optical density (OD) of wells at 590 nm was measured before and after 24 h incubation at 37 °C. All experiments were performed in triplicate. The inhibition rate was calculated as the following equation.
Where ODs is the optical density of 1-O-alkylglycerol samples, ODc is the optical density of control.
2.8. Antifungal activity
The antifungal effects of C8Gly1, C12Gly1, C16Gly1 against the pathogens Aspergillus flavus (A. flavus), Botryosphaeria dothidea (B. dothidea), Monilia fructigena (M. fructigena), and Phytophthora capsica (P. capsica) were evaluated by the method of in vitro plate [21]. 1-O-alkylglycerols were weighed and dissolved in DMSO solutions (containing 1 % Tween 80) to the concentration of 100 mg/mL, further dilution by mixing with PDA medium (200 mL) to the concentrations of 50, 100, 200, and 400 μg/mL, and then shake the medium and pour into a Petri dish. After solidified, the activated fungi were punched at the edge of the hyphae with a hole punch of 5 mm in diameter to form a fungus block, and the fungus block was inoculated to the center of the medium with an inoculation knife, sealed with a parafilm, and then placed in a constant temperature at 28 °C. Each experiment was repeated three times. The growth inhibition rate was calculated as the following formula:
Where Dc means the diameter of the control group, De means the diameter of the experimental group.
3. Results and discussion
3.1. Synthesis and structural characterization of 1-O-alkylglycerols
There is currently no effective direct synthesis of 1-O-alkylglycerols with good yield and regioselectivity from glycerol and alcohol. As a result, to obtain pure molecules, two steps are typically needed: first, the synthesis of protected or manipulated glycerol ethers, and second, the deprotection by acids. The most frequent reactant etherified by activated (halogenated, mesylated, or tosylated) alcohol is solketal (1,2-isopropylideneglycerol). We avoided using organic solvent and chose solketal and readily available halogenated alkanes as reactants. At 35–40 °C for 4 h, the solketal was etherified with brominated alkanes using tetrabutylammonium bromide (TBAB) as a phase transfer catalyst and KOH as a base, and the acetal was hydrolyzed using an aqueous p-toluenesulfonic acid solution to provide 1-O-alkylglycerols. The isolated yield of 1-O-alkylglycerols rises with alkyl chain length, from 71.3 % (C8Gly1), 84.7 % (C12Gly1), to 89.8 % (C16Gly1). The continuously increasing yields should be attributed to decreased water solubility as alkyl chain length increases during the liquid-liquid extraction.
The chemical structures of all 1-O-alkylglycerols were confirmed by recording their 1H NMR, 13C NMR, FTIR, and mass spectra. The proton numbers followed the corresponding structure of 1-O-alkylglycerols, except for these in hydroxyl groups that were not found. The results are in agreement with the literature [20]. However, the FTIR spectra confirmed the existence of hydroxyl group. The characteristic peaks at 3364-3413 cm−1 corresponded to the stretching vibration of O–H, and the bending vibration of secondary and primary alcohols were found at 1116 cm−1 and 1045-1060 cm−1. Mass spectrum of C8Gly1 displayed the pseudo-molecular ion peak at m/z 186.22 [M − H2O]+, which may be due to the cyclization reaction to remove a water molecule. While the most abundant ion peak of C12Gly1 and C16Gly1 were their [M+Na]+ peaks. The mass spectrometry results indicated that the molecular weights were consistent with their molecular formula.
3.2. HPLC-ELSD characterization
HPLC-ELSD was further used to assess the purity of synthesized 1-O-alkylglycerols. The retention time of C16Gly1 standard with 98 % purity was 5.16 min, as shown in Fig. 3, and the retention time of synthesized C16Gly1 was a minor difference from the standard, suggesting the correctness of the product. As the molecule's polarity increases, the retention time of C12Gly1 and C8Gly1 decreased to 3.40- and 2.49 min, respectively. Furthermore, the absence of impurities in the HPLC-ELSD peaks demonstrated that the 1-O-alkylglycerols were of excellent purity.
Fig. 3.
HPLC-ELSD characterization of the three 1-O-alkylglycerols and C16Gly1 standard. The purity of C16Gly1 standard is 98 %, which indicates that the three 1-O-alkylglycerols are also in high purity.
3.3. CMC values
The critical micelle concentration (CMC), defined as the minimum concentration of a surfactant at which micelles begin to form, is an essential parameter for a surfactant. The fluorescence method by measuring the fluorescence of pyrene was used to determine the CMC of C8Gly1, C12Gly1, and C16Gly1 [22,23], and the ratios I1/I3 versus the 1-O-alkylglycerol concentrations were plotted in Fig. 4A, B, and 4C. The curves showed a sharp decrease in the quotient intensities of vibration peaks when micelles were formed (i.e., at the CMC). The CMC values are regarded as the surfactant concentrations that correlate to the sharp decrease [24]. Finally, the CMC values of C8Gly1, C12Gly1, and C16Gly1 were 1.65 mmol/L (Figs. 4A), 0.33 mmol/L (Figs. 4B), and 0.23 mmol/L (Fig. 4C), with a considerable drop as the alkyl chain length increased from C8 to C12 and C16 (Fig. 4D). The longer alkyl chain length resulted in a lower CMC owing to the surfactant's more hydrophobic nature.
Fig. 4.
Plots of pyrene I1/I3 versus 1-O-alkylglycerol concentration for (A) C8Gly1, (B) C12Gly1, and (C) C16Gly1. Red line described the Boltzmann-type sigmoid, while black lines represented the first-order derivative variation curves. Dashed line indicated the CMC value. D shows the relationship between CMC and alkyl chain length. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Ricciardi et al. reported a comparable CMC value (1.12 mmol/L) of C8Gly1 measured by UV absorption of pyrene [25]. However, De Paula et al. [19] and Bigot et al. [26] reported that the determined CMC values of C8Gly1 were 6 mmol/L and 7.1 mmol/L, respectively. These findings suggested that these two approaches will produce different outcomes for 1-O-alkylglycerols with shorter alkyl chain lengths, such as those less than 8. However, the higher intermolecular hydrophobic interaction will promote aggregation in water and boost the accuracy of CMC determination as alkyl chain length increases, as evidenced by the identical CMC values of C10Gly1 (0.40 mmol/L) [19] and C12Gly1 (0.33 mmol/L).
3.4. Dynamic surface tension of 1-O-alkylglycerol
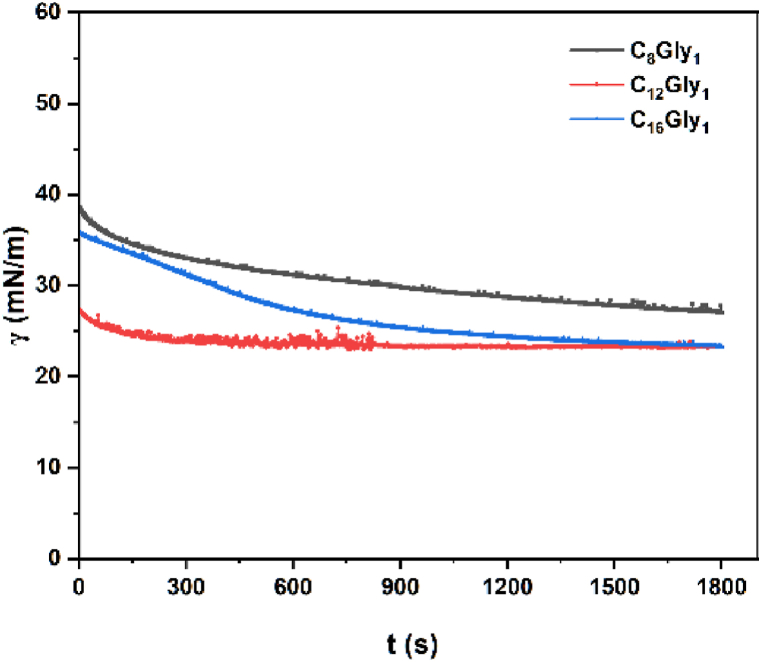
Dynamic surface tension of low molecular weight surfactants has been a subject of active research, as it is relevant to a variety of processes, including emulsification to printing, coating, and mineral flotation [27]. The pendant drop method was used for measuring surface tension over hundreds or even thousands of seconds. The pitfalls associated with long measurement times and waiting for equilibrium to be attained were shown in Fig. 5. In this experiment, the surface tension of a droplet of three 1-O-alkylglycerol solutions at the concentration of CMC was measured over time. From Fig. 5, the dynamic surface tension of the C12Gly1 droplet decreased smoothly from 27.26 mN/m to its equilibrium surface tension of 23.37 mN/m in 300 s, implying the adsorption of C12Gly1 molecules onto the freshly created droplet surface. The higher initial surface tension (35.79 mN/m) and longer equilibrium time (about 1400 s) of C16Gly1 droplet than that of C12Gly1 indicated the slower diffusion of C16Gly1 from the bulk phase to the surface. The calculated surface tension of C8Gly1 is 27.05 mN/m after 1800 s, demonstrating its lower ability to decrease surface tension than C12Gly1 and C16Gly1.
Fig. 5.
The dynamic surface tension as a function of time for C8Gly1, C12Gly1, and C16Gly1 solution at the concentration of CMC and 25 °C.
3.5. Z-average mean diameter, PDI, and Zeta-potential
As surfactants, 1-O-alkylglycerols will aggregate to form micelles in water; therefore, the key parameters, Z-average mean diameter, PDI, and Zeta-potential, at CMC conditions were measured and summarized in Table 1. The stronger hydrophobicity of C12Gly1 and C16Gly1 likely caused the formation of bigger aggregates, which resulted in the micelle size increasing dramatically with the length of the alkyl chain. The PDI showed C8Gly1 aggregates scattered in water with extremely excellent uniformity [28], but not for C12Gly1 and C16Gly1, also suggesting the presence of both large and tiny molecular aggregates. Furthermore, as the length of the alkyl chain rose, the aggregates of 1-O-alkylglycerols declined in the absolute Zeta-potential. The higher Zeta-potential in the magnitude of C8Gly1 also facilitated the smaller aggregated and better dispersion uniformity. Given that the hydrophobic chain length of C8Gly1 is shorter than that of other compounds, this might have encouraged interaction with more water dipoles, contributing to the higher Zeta-potential value.
Table 1.
The Z-average mean diameter (Z-average), PDI, and zeta-potential of C8Gly1, C12Gly1 and C16Gly1 in aqueous solution at the concentration of CMC.
| Sample | Z-Average (nm) | PDI | Zeta-potential (mV) | |
|---|---|---|---|---|
| C8Gly1 | 164.6 ± 24.5 | 0.197 ± 0.048 | −31.8 ± 1.3 | |
| C12Gly1 | 391.4 ± 12.1 | 0.311 ± 0.049 | −10.2 ± 0.9 | |
| C16Gly1 | 693.3 ± 8.5 | 0.349 ± 0.017 | −5.4 ± 1.9 |
3.6. Antibacterial activity
To investigate the antibacterial activity of 1-O-alkylglycerols, the Gram-positive S. aureus and Gram-negative E. coli, two main pathogenic bacteria in food pollution, were studied by the microbroth dilution method. As shown in Fig. 6, C8Gly1 and C12Gly1 both displayed antibacterial activities only against S. aureus at concentrations lower than 800 μg/mL, demonstrating the selectivity between S. aureus and E. coli. C12Gly1 displayed 73.3 % inhibition at 25 μg/mL, and the inhibition rate did not increase significantly with the concentration increasing. While C8Gly1 had 87.1 % and 93.5 % inhibition at 400 and 800 μg/mL, respectively, showing more robust antibacterial activity than C12Gly1. For E. coli, only C8Gly1 showed 81.3 % inhibition when the concentration increased to 800 μg/mL. C16Gly1 showed no inhibition activity against S. aureus and E. coli at all concentrations (data not shown).
Fig. 6.
Antibacterial activity of (a) C8Gly1 against S. aureus, (b) C12Gly1 against S. aureus, (c) C8Gly1 against E. coli, and (d) C12Gly1 against E. coli.
The stronger inhibition of C12Gly1 against S. aureus indicated that Gram-positive bacteria were more susceptible than Gram-negative bacteria, which is in accordance with the report [3]. The 1-monolaurin, a common food antibacterial agent with structural similarity to C12Gly1, also showed high potency against Gram-positive bacteria, indicating they may display similar antibacterial mechanism. The higher potency of C12Gly1 against Gram-positive bacteria could be due to their differences in cell wall structure compared to Gram-negative bacteria, which is lipo-oligosaccharides in the outer membrane that are susceptible to hydrophobic substances [5]. The better chemical stability of C12Gly1 owing to its ether bond is an advantage that can maintain a longer effective period. In addition, the lower polarity of C12Gly1, endowing it with better water solubility, is another advantage that can expand its application fields.
3.7. Antifungal activity
Four phytopathogenic fungi, A. flavus, B. dothidea, M. fructigena, and P. capsicum, were selected for preliminary in vitro antifungal activity screening since they are all fungi that need to be controlled during the crop growth or after harvest. Fig. 7 illustrates the growth inhibition at 50, 100, 200, and 400 μg/mL by C8Gly1, C12Gly1, and C16Gly1, respectively. Generally, C8Gly1 and C12Gly1 showed antifungal activity only at the concentration of 400 μg/mL, and C16Gly1 showed no growth inhibition at all, which is accordant with the antibacterial activity aforementioned. C8Gly1 had the inhibition rates against A. flavus, B. dothidea, M. fructigena, and P. capsicum of 81.3 %, 90.9 %, 100 %, and 64.7 %, and the inhibition rates of C12Gly1 were 58.8 %, 39.6 %, 97.0 %, and 95.3 % at 400 μg/mL, respectively. C8Gly1 showed broader antifungal activity than C12Gly1. The antifungal mechanism of 1-O-alkylglycerols may be comparable to that of common surfactants, which destroy the fungal cell membranes [29]. The 1-O-alkylglycerols adsorbed and passed through the cell well into the cell membrane. However, changes in cell structure amongst fungi resulted in disparities in antifungal activity.
Fig. 7.
Antifungal activity of C8Gly1, C12Gly1, and C16Gly1 against (A) A. flavus, (B) B. dothidea, (C) M. fructigena, (D) P. capsicum, and their inhibition on the mycelial growth diameter of (a) A. flavus, (b) B. dothidea, (c) M. fructigena, (d) P. capsicum at the concentrations of 50, 100, 200, and 400 μg/mL. CK represents the control sample.
B. dothidea, M. fructigena, and P. capsicum cause loss in the field and in the postharvest stage during storage, shipping and marketing [[30], [31], [32]]. Applying chemical fungicides remains the most effective measure to control fungi due to their efficiency and stability. However, prolonged usage of chemical fungicides may result in pathogen resistance and environmental pollution. Therefore, it is urgently required to develop alternative fungicides. 1-O-alkylglycerols are an important potential alternative owing to their effective antifungal activities and biocompatibility. The highly potent antifungal activity of C8Gly1 against B. dothidea and M. fructigena, and C12Gly1 against M. fructigena and P. capsicum demonstrates they can be novel potential antifungal compounds. On the other hand, 1-O-alkylglycerols are amphiphilic surfactants that assemble intro vesicles that can be applied as encapsulation carriers, just as Gopinath et al. reported [33]. Madhusudhan et al. [34] reported the carbamazepine loaded O/W nanoemulsions stabilized by 1-O-alkylglycerol/lecithin complex and proved the potential application of 1-O-alkylglycerol stabilized nanoemulsions. Therefore, the antifungal C8Gly1 and C12Gly1 could be fabricated as the nanoencapsulation carriers for fungicides in plant protection or food preservation, which can increase the fungicidal activity by the potential synergetic effect.
4. Conclusions
In this study, we synthesized three 1-O-alkylglycerols with the isolated yields of 71.3 %–89.9 % and evaluated their surface property, antibacterial, and antifungal activities. Surface properties revealed that their CMC values were 1.65 mmol/L (C8Gly1), 0.33 mmol/L (C12Gly1), and 0.23 mmol/L (C16Gly1). The surface tension of C12Gly1 and C16Gly1 aqueous solutions can be reduced to 23.37 mN/m, while C8Gly1 only 35.79 mN/m. C8Gly1 could form a highly stable and uniform nanoscale dispersion in water. Furthermore, C8Gly1 and C12Gly1 displayed strong antibacterial activity against S. aureus, indicating the selectivity to Gram-positive bacteria. C8Gly1 and C12Gly1 also potently inhibited the growth of B. dothidea, M. fructigena, and P. capsicum at 400 μg/mL. As a result, the C8Gly1 and C12Gly1 can be used as potential antimicrobial agents in the fields of food and agriculture.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Liangliang Shi: Formal analysis, Investigation, Writing – original draft. Chenyue Jia: Formal analysis, Investigation. Jiangtao Feng: Formal analysis, Methodology. Weinong Zhang: Formal analysis. Junbo He: Funding acquisition, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
There's no conflict of interest in this work.
Acknowledgments
This work was supported by the Science and Technology Research Project of Educational Commission of Hubei Province of China (D20221602).
References
- 1.Kabara J.J., Vrable R., Lie Ken Jie M.S.F. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- 2.Churchward C.P., Alany R.G., Snyder L.A.S. Alternative antimicrobials: the properties of fatty acids and monoglycerides. Crit. Rev. Microbiol. 2018;44:561–570. doi: 10.1080/1040841X.2018.1467875. [DOI] [PubMed] [Google Scholar]
- 3.Ved H.S., Gustow E., Mahadevan V., Pieringer R.A. Dodecylglycerol. A new type of antibacterial agent which stimulates autolysin activity in Streptococcus faecium ATCC 9790. J. Biol. Chem. 1984;259:8115–8121. [PubMed] [Google Scholar]
- 4.Kabara J.J., Swieczkowski D.M., Conley A.J., Truant J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X., Liu W., Zhao M., Huang Y., Feng F. Glycerol monolaurate beyond an emulsifier: synthesis, in vivo fate, food quality benefits and health efficacies. Trends Food Sci. Technol. 2022;127:291–302. [Google Scholar]
- 6.Iannitti T., Palmieri B. An update on the therapeutic role of alkylglycerols. Mar. Drugs. 2010;8:2267–2300. doi: 10.3390/md8082267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnusson C.D., Haraldsson G.G. Ether lipids. Chem. Phys. Lipids. 2011;164:315–340. doi: 10.1016/j.chemphyslip.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese P.T., Jordan K., Cederberg H., Brohult J. Some biological actions of alkylglycerols from shark liver oil. J. Alternative Compl. Med. 1998;4:87–99. doi: 10.1089/acm.1998.4.1-87. [DOI] [PubMed] [Google Scholar]
- 9.Hallgren B., Larsson S. The glyceryl ethers in man and cow. JLR (J. Lipid Res.) 1962;3:39–43. [Google Scholar]
- 10.Homma S., Millman I., Yamamoto N. A serum factor for macrophage activation after in vitro dodecylglycerol treatment of mouse lymphocytes. Immunol. Cell Biol. 1990;68:137–142. doi: 10.1038/icb.1990.19. [DOI] [PubMed] [Google Scholar]
- 11.Oh S.Y., Jadhav L.S. Effects of dietary alkylglycerols in lactating rats on immune responses in pups. Pediatr. Res. 1994;36:300–305. doi: 10.1203/00006450-199409000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hichami A., Duroudier V., Leblais V., Vernhet L., Le Goffic F., Ninio E., Legrand A. Modulation of platelet-activating-factor production by incorporation of naturally occurring 1-O-alkylglycerols in phospholipids of human leukemic monocyte-like THP-1 cells. Eur. J. Biochem. 1997;250:242–248. doi: 10.1111/j.1432-1033.1997.0242a.x. [DOI] [PubMed] [Google Scholar]
- 13.Pedrono F., Martin B., Leduc C., Le Lan J., Saïag B., Legrand P., Moulinoux J.-P., Legrand A.B. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr. Cancer. 2004;48:64–69. doi: 10.1207/s15327914nc4801_9. [DOI] [PubMed] [Google Scholar]
- 14.Deniau A.-L., Mosset P., Le Bot D., Legrand A.B. Which alkylglycerols from shark liver oil have anti-tumour activities? Biochimie. 2011;93:1–3. doi: 10.1016/j.biochi.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Momha R., Kuete V., Pagès J.-M., Pegnyemb D.E., Mosset P. Synthesis and biological evaluation of four new ricinoleic acid-derived 1-O-alkylglycerols. Mar. Drugs. 2020;18:113. doi: 10.3390/md18020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brissette J.L., Cabacungan E.A., Pieringer R.A. Studies on the antibacterial activity of dodecylglycerol. Its limited metabolism and inhibition of glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans BHT. J. Biol. Chem. 1986;261:6338–6345. [PubMed] [Google Scholar]
- 17.Haynes M.P., Buckley H.R., Higgins M.L., Pieringer R.A. Synergism between the antifungal agents amphotericin B and alkyl glycerol ethers. Antimicrob. Agents Chemother. 1994;38:1523–1529. doi: 10.1128/aac.38.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ved H.S., Gustow E., Pieringer R.A. The involvement of the proteinase of Streptococcus faecium ATCC 9790 in the stimulation of its autolysin activity by dodecylglycerol. J. Biol. Chem. 1984;259:8122–8124. [PubMed] [Google Scholar]
- 19.de Paula F.H.M., de Freitas F.A., Nunes D.G., Iglauer S., Gramatges A.P., Nascimento R.S.V., Lachter E.R. Alkyl glyceryl ethers as water-based lubricant additives in mixtures with xanthan gum. Colloids Surf. A Physicochem. Eng. Asp. 2022;634 [Google Scholar]
- 20.Halldorsson A., Thordarson P., Kristinsson B., Magnusson C.D., Haraldsson G.G. Lipase-catalysed kinetic resolution of 1-O-alkylglycerols by sequential transesterification. Tetrahedron: Asymmetry. 2004;15:2893–2899. [Google Scholar]
- 21.Zhou Y., Zhang S., Cai M., Wang K., Feng J., Xie D., Feng L., Peng H., He H. Design, synthesis, and antifungal activity of 2,6-Dimethyl-4-aminopyrimidine hydrazones as PDHc-E1 inhibitors with a novel binding mode. J. Agric. Food Chem. 2021;69:5804–5817. doi: 10.1021/acs.jafc.0c07701. [DOI] [PubMed] [Google Scholar]
- 22.Fu J., Cai Z., Gong Y., O'Reilly S.E., Hao X., Zhao D. A new technique for determining critical micelle concentrations of surfactants and oil dispersants via UV absorbance of pyrene. Colloids Surf. A Physicochem. Eng. Asp. 2015;484:1–8. [Google Scholar]
- 23.Aguiar J., Carpena P., Molina-Bolívar J.A., Carnero Ruiz C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003;258:116–122. [Google Scholar]
- 24.Mitsionis A.I., Vaimakis T.C. Estimation of AOT and SDS CMC in a methanol using conductometry, viscometry and pyrene fluorescence spectroscopy methods. Chem. Phys. Lett. 2012;547:110–113. [Google Scholar]
- 25.Ricciardi M., Cucciniello R., Barrault J., Faggiano A., Capacchione C., Proto A. A step towards bio-surfactants: monoalkylglyceryl ethers synthesis through glycidol alcoholysis with long-chain alcohols catalyzed by Al(OTf)3. Sustainable Chemistry and Pharmacy. 2020;17 [Google Scholar]
- 26.Bigot S., Bricout H., Suisse I., Mortreux A., Castanet Y. Synthesis and surface properties of glycerol based C8 chain monoethers. Ind. Eng. Chem. Res. 2011;50:9870–9875. [Google Scholar]
- 27.Berry J.D., Neeson M.J., Dagastine R.R., Chan D.Y.C., Tabor R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015;454:226–237. doi: 10.1016/j.jcis.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 28.He J., Wang M., Zhu P., Zhang H., Hu C., Zhang W. Novel polyglycerol-10 dialdehyde mediated cross-linking of sodium caseinate: preparation, characterization, and improved emulsifying properties. Colloids Surf. A Physicochem. Eng. Asp. 2022;647 [Google Scholar]
- 29.Tawfik S.M., Zaky M.F., Mohammad T.G.M., Attia H.A.E. Synthesis, characterization, and in vitro antifungal activity of anionic and nonionic surfactants against crop pathogenic fungi. J. Ind. Eng. Chem. 2015;29:163–171. [Google Scholar]
- 30.Li J., Fu S., Fan G., Li D., Yang S., Peng L., Pan S. Active compound identification by screening 33 essential oil monomers against Botryosphaeria dothidea from postharvest kiwifruit and its potential action mode. Pestic. Biochem. Physiol. 2021;179 doi: 10.1016/j.pestbp.2021.104957. [DOI] [PubMed] [Google Scholar]
- 31.Aiello D., Restuccia C., Stefani E., Vitale A., Cirvilleri G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 2019;149:83–89. [Google Scholar]
- 32.Wang B., Li P., Yang J., Yong X., Yin M., Chen Y., Feng X., Wang Q. Inhibition efficacy of Tetradium glabrifolium fruit essential oil against Phytophthora capsici and potential mechanism. Ind. Crop. Prod. 2022;176 [Google Scholar]
- 33.Gopinath D., Ravi D., Rao B.R., Apte S.S., Rambhau D. 1-O-Alkylglycerol vesicles (Algosomes): their formation and characterization. Int. J. Pharm. 2002;246:187–197. doi: 10.1016/s0378-5173(02)00397-6. [DOI] [PubMed] [Google Scholar]
- 34.Madhusudhan B., Rambhau D., Apte S.S., Gopinath D. 1-O-alkylglycerol stabilized carbamazepine intravenous o/w nanoemulsions for drug targeting in mice. J. Drug Target. 2007;15:154–161. doi: 10.1080/10611860601141150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.