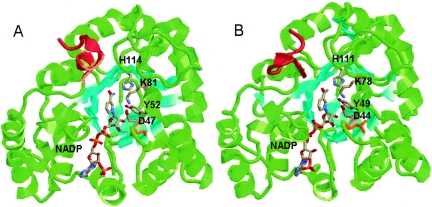
FIG. 3.
(A) Crystal structure of C. tenuis XR with bound NADPH (1K8C). (B) Homology model of N. crassa XR with bound NADPH, built using the Insight II and MOE programs. The β-sheets are colored cyan to aid in the visualization of the (α/β)8 barrel. The catalytic tyrosine, lysine, and aspartate, as well as the bound cofactors and conserved histidine, are colored by atom type. The C-terminal regions involved in dimerization are colored red. Cys23 of C. tenuis XR (A) and Leu20 of N. crassa XR (B) are colored orange.