Abstract
Importance
Results from high-profile randomized controlled trials (RCTs) are routinely reported through press release months prior to peer-reviewed publication. There are potential benefits to press releases (e.g., knowledge dissemination, ensuring regulatory compliance), but also potential drawbacks (e.g., selective reporting, positive “spin”).
Objective
To characterize the practice of press release predating the publication of a drug-related RCT in a peer-reviewed journal (“preemptive press release”), including factors associated with this practice.
Design, Setting, and Participants
We systematically reviewed all RCTs of medications published between 2015 and 2019 in the New England Journal of Medicine (NEJM), Journal of the American Medical Association (JAMA), and Lancet. Press releases were identified using a systematic search of the grey literature (e.g., press release databases, study sponsor websites). An RCT was considered to have a preemptive press release if the press release was published at least three months (90 days) prior to the date of publication in a peer-reviewed journal.
Main Outcomes and Measures
Presence of preemptive press release, defined as a press-release at least 90 days prior to the date of publication in a peer-reviewed journal. As secondary measures for dissemination, we also assessed citation count and Altmetric score.
Results
We identified 988 RCTs, of which 172 (17%) had a press release published at least 90 days before the date of peer-reviewed publication. Press releases were published a median of 246 days (interquartile range [IQR] 169–366 days) before publication in a peer-reviewed journal. In the multivariable logistic regression model, the strongest predictor of having a preemptive press release was funding by a pharmaceutical company (odds ratio 13, 95% CI 7, 25). Approximately 85% of RCTs with preemptive press releases had a positive primary outcome and, concordantly, 81% of the corresponding press releases had a positive headline. Multivariable regression models identified studies with a preemptive press release had a similar Altmetric score (median − 15, 95% CI − 33, 12) and higher median citation count (median 22 [95% CI 10 to 33] compared to studies without a preemptive press release.
Conclusions and Relevance
Preemptive press releases were common, most often issued for trials funded by a pharmaceutical company, and typically preceded publication in a peer-reviewed journal by approximately eight months.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08313-1.
INTRODUCTION
Randomized controlled trials (RCTs) are one of the most powerful tools to generate rigorous and reliable evidence to inform clinical decision-making. Prior to publication in a medical journal, RCTs typically undergo peer review by content experts and statistical reviewers. Peer review allows for an assessment of the study’s design and conduct; during the process, errors, biases, and incorrect assumptions can be identified and corrected prior to publication. Once a manuscript is accepted for publication, there is a media embargo date prior to which journals stipulate that the publication cannot be shared or disseminated.
On the date of publication, researchers, funding organizations, academic medical centers, pharmaceutical companies, and/or government institutions may distribute a press release to the media.1 These statements often include a brief synopsis of the study, summarize the results, and comment on the implications of the research. One purpose of a press release is to ensure regulatory compliance for studies conducted or funded by industry; another purpose is to attract media attention. Papers with press releases are twice as likely to receive website views, be downloaded, and be cited than papers that do not have associated press releases.2
While leveraging press releases for media attention enhances the reach and application of study results, the practice introduces the potential for selective reporting of trial results.3 When the press release occurs on the same date as the publication, clinicians can simply access the study itself to understand and contextualize its findings before applying them to practice. However, when the press release precedes the date of publication, this is impossible. Known examples of the preemptive and incomplete distribution of results via press release include the PIONEER 6, REDUCE-IT, and SPRINT trials.4 Issued in 2015, the press release for the SPRINT trial reported that in comparison to a goal systolic blood pressure (BP) of 140 mmHg, more intensive BP management resulted in lower all-cause mortality.5 However, the press release did not indicate the significant harms associated with this management approach, including hypotension, renal failure, and syncope, that were apparent in the peer-reviewed manuscript published months later.5, 6 The initial enthusiasm for adopting intensive BP management waned once clinicians had access to the published paper and raised concerns about the accuracy of the content included in the preemptive press release.4
Instances of preemptive and incomplete distribution of study results via press releases invite questions about how often preemptive press releases occur, the factors associated with this practice, and how the content of the press release contrasts with the study results.7 The objective of our study was to characterize the practice of press release predating the publication of an RCT in a peer-reviewed journal (“preemptive press release”), including factors associated with this practice and how preemptive press releases affect knowledge dissemination.
METHODS
Study Population
All RCTs of medications published in the New England Journal of Medicine (NEJM), Journal of the American Medical Association (JAMA), or Lancet between January 1, 2015, and December 31, 2019, were systematically reviewed. These journals were selected because they commonly publish RCTs. RCTs published after January 1, 2020, were not included because the publishing landscape markedly changed as a result of the COVID-19 pandemic, including the use of pre-print servers to share results prior to publication. Using MEDLINE, we identified all research articles published in these journals during the study time period, excluding review articles, research letters, letters to the editor, and editorials. The title and abstract of the remaining articles were reviewed independently by two study members (MF, UP) to identify and exclude non-randomized studies, duplicate publications, or RCTs that did not include medication. Disagreements were resolved through consensus. Figure 1 describes the study selection process. Research ethics board approval was not required because all data sources used for this study were publicly available.
Figure 1.
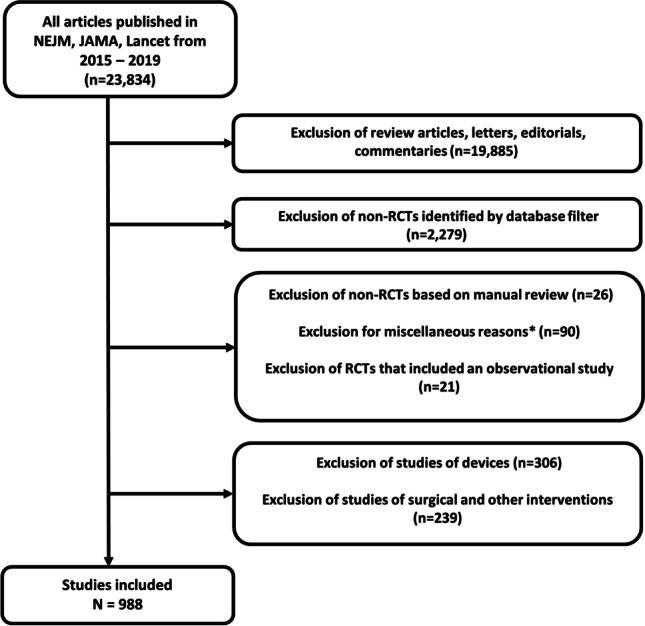
Study flow diagram. Abbreviations: JAMA: Journal of the American Medical Association, NEJM: New England Journal of Medicine, RCT: randomized controlled trial. * “Miscellaneous reasons” included trials that were secondary analyses of the trial or long-term/follow-up results from the initial trial.
Data Collection for Individual RCTs
For each RCT, the abstract was then reviewed by a study member (ARaissi, UP, MB). An initial data collection form was piloted using 15 abstracts and then revised based on consensus (MF, UP, CAS). The following data were collected for each RCT: journal, disease area, sample size, blinding, funding source, comparator type (e.g., placebo), outcome type (i.e., surrogate outcome or not), and primary outcome result (i.e., positive, negative, or neutral). A study’s outcome was considered positive if the point estimate for the primary outcome identified a benefit and the 95% confidence intervals excluded the null. The study outcome was considered negative if the point estimate for the primary outcome identified harm and the 95% confidence intervals excluded the null. The study outcome was considered neutral if the point estimate included the null. For non-inferiority trials, the study outcome was considered positive if the study was non-inferior or both non-inferior and superior (see Appendix). If there was uncertainty among the three reviewers on an appropriate categorization of the data, a fourth study member (MF) reviewed the abstract to provide the final decision.
Press Release Data Collection
Press releases were identified through a systematic search of the grey literature. An RCT was considered to have a “preemptive press release” if the press release was published at least 90 days prior to the date of peer-reviewed publication. Study members (MB, SM, UP) followed a uniform and systematic set of steps in order to identify press releases associated with drug-related RCTs. If the RCT was funded by a pharmaceutical company, the first step was to find the press release using the primary funder’s website. If not found on the website, the FACTIVA database was used. FACTIVA is an international news database that aggregates data from more than 32,000 licensed and free sources.8 We searched using the following terms: name of drug being studied, name of the study’s sponsor, the study’s acronym (when available), and the ClinicalTrials.gov identifier. If a press release was not found on FACTIVA, a Google search was run using the same search terms. If an RCT was not industry-funded, FACTIVA and Google databases were searched using the aforementioned strategy. We initially captured the following data during the press release identification process: number of press releases found, publication date of the earliest press release, and website/database from which the press release was found.
A data collection form for reviewing the preemptive press releases was piloted using 25 press-releases and then revised based on consensus (UP, MB, SM, CAS). The following information was collected from these press releases: study interpretation tone (positive, negative, neutral), primary endpoint achieved (yes, no, inconclusive, not discussed), and extent of focus on a secondary endpoint (one or more paragraphs, less than one paragraph, no mention). Concordance between the press release and study results was extrapolated by comparing the proportion of press releases reporting a positive outcome (primary endpoint achieved, positive interpretation tone) and the proportion of RCT results reporting a positive outcome. The text of the headline and press release were downloaded and saved in a CSV file to allow for natural language processing. All preemptive press releases were reviewed by two investigators and disagreement was resolved through consensus.
Natural Language Processing
Natural language processing (NLP) was used to summarize the overall tone of the press release headlines in an objective manner. Specifically, sentiment analysis was conducted using a neural network-based technique to classify headlines as being either positive, negative, or neutral. This was conducted using RoBERTa, a transformer-based model that has been pre-trained on a large corpus of English text in a self-supervised fashion and routinely applied in sentiment analysis.9, 10
Statistical Analysis
Descriptive statistics were used to describe details related to the included clinical trials, including results related to the study and press release tone. A multivariable logistic regression model was used to identify study-level variables that were associated with preemptive press release. The following variables were selected a priori: year of publication, disease area, funding source, and whether the study had a positive, negative, or neutral primary outcome. Citation counts were compared between the articles with and without preemptive press releases; these data were obtained using the CrossRef API via the rcrossref R package and merged using the Digital Object Identifier (DOI) of each article. Because the residuals were not normally distributed for citation count, we did not pursue linear regression and instead used quantile regression, which models the median value (quantreg package in R). Additionally, Altmetric scores were used to further quantify the amount of attention received by articles with and without preemptive press releases. Altmetric scores are weighted counts derived from an algorithm that incorporates various factors such as the reach of a source (for example, a newspaper article is credited with having further reach than a blog post), volume (number of times the article is mentioned), and mention by author (an article shared online by the author gathers more attention than one shared by another source).11 The Altmetric data were obtained through the Altmetric Details page using the rAltmetric R package. Because the residuals were not normally distributed for Altmetric, we did not pursue linear regression and instead used quantile regression. The supplementary appendix provides additional details on these techniques. All analyses were conducted using R version 3.1.2.5.
RESULTS
Between January 1, 2015, and December 31, 2019, there were 988 unique RCTs of medications published in NEJM, JAMA, and Lancet. These RCTs were primarily in the areas of oncology (n = 179, 18%), cardiovascular disease (n = 166, 17%), and infectious disease (n = 154, 16%; Table 1). Approximately half of the included studies (n = 482, 49%) were funded by pharmaceutical companies; 498 (50%) studies were double-blinded; 495 (50%) were placebo-controlled; 434 (44%) had a surrogate outcome as the primary endpoint; and 653 (66%) studies reported a positive primary outcome.
Table 1.
Baseline Characteristics of Included Randomized Controlled Trials
| Studies with preemptive press release | Studies without preemptive press release | |
|---|---|---|
| n = 172 | n = 816 | |
| Trial sample size (IQR) | 630 (331, 1474) | 582 (260, 1716) |
| Journal (n, %) | ||
| NEJM | 89 (51.7) | 373 (45.7) |
| JAMA | 10 (5.8) | 176 (21.6) |
| Lancet | 73 (42.4) | 267 (32.7) |
| Year of publication (n, %) | ||
| 2015 | 27 (15.7) | 161 (19.7) |
| 2016 | 35 (20.3) | 142 (17.4) |
| 2017 | 44 (25.6) | 153 (18.8) |
| 2018 | 32 (18.6) | 148 (18.1) |
| 2019 | 34 (19.8) | 212 (26.0) |
| Disease area (n, %) | ||
| Cardiovascular disease | 22 (12.8) | 144 (17.6) |
| Endocrinology | 7 (4.1) | 39 (4.8) |
| Infectious disease | 19 (11.0) | 135 (16.5) |
| Neurology | 15 (8.7) | 53 (6.5) |
| Oncology | 48 (27.9) | 131 (16.1) |
| Psychiatry | 2 (1.2) | 17 (2.1) |
| Respirology | 13 (7.6) | 60 (7.4) |
| Other | 46 (26.7) | 237 (29.0) |
| Funding source (n, %) | ||
| Pharma | 156 (90.7) | 326 (40.0) |
| Non-pharma | 11 (6.4) | 410 (50.2) |
| Combined | 5 (2.9) | 80 (9.8) |
| Blinding (n, %) | ||
| Double | 96 (55.8) | 402 (49.3) |
| Single | 3 (1.7) | 64 (7.8) |
| Unblinded (open label) | 73 (42.4) | 350 (42.9) |
| Comparator (n, %) | ||
| Placebo/sham | 88 (51.2) | 407 (49.9) |
| Active comparator | 77 (44.8) | 348 (42.6) |
| Other | 7 (4.1) | 61 (7.5) |
| Surrogate marker endpoint (n, %) | ||
| Yes | 97 (56.4) | 337 (41.3) |
| No | 75 (43.6) | 479 (58.7) |
| Primary endpoint result (n, %) | ||
| Negative | 3 (1.7) | 19 (2.3) |
| Neutral | 23 (13.4) | 290 (35.5) |
| Positive | 146 (84.9) | 507 (62.1) |
Abbreviations: IQR, interquartile range denotes the first and third quartile; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine
Approximately one-fifth (n = 172, 17%) of the RCTs had a preemptive press release. Press release characteristics can be found in Table 2. The preemptive press releases were published a median of 246 days (interquartile range [IQR] 169–366) in advance of publication in a peer-reviewed journal. Most press releases (83%) were located using the primary funder’s website. Approximately 85% of RCTs with preemptive press releases had a positive primary outcome and, concordantly, 81% of the corresponding press releases had a positive headline (based on manual review). Our NLP sentiment analysis of the preemptive press release headlines found that 61% of the headlines had a positive sentiment, 36% had a neutral sentiment, and 2% had a negative sentiment.
Table 2.
Details of Preemptive Press Release
| n = 172 | |
|---|---|
| Timing prior to peer-review publication | 246 days (IQR 169, 366) |
| Press release source (n, %) | |
| Funder website | 143 (83) |
| 20 (12) | |
| Factiva | 6 (4) |
| Other | 3 (2) |
| Tone of press release headline* (n, %) | |
| Positive | 139 (81) |
| Negative | 4 (2) |
| Neutral | 27 (16) |
| Primary outcome result (n, %) | |
| Positive | 146 (85) |
| Negative | 3 (2) |
| Neutral | 23 (13) |
| Discussion of secondary endpoints (n, %) | |
| One or more paragraph | 55 (32) |
| Less than one paragraph | 36 (21) |
| Not discussed | 79 (46) |
Abbreviations: IQR, interquartile range, denotes the first and third quartile. *Note: These results reflect manual human interpretation of press release headlines conducted by the study team; two studies had a missing headline and press-release
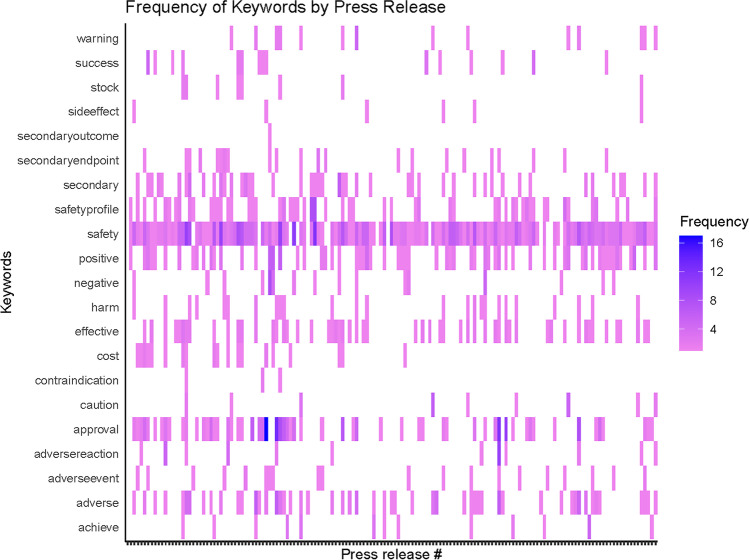
One or more paragraphs were allocated to secondary endpoints in 55 (32%) of the press releases. NLP analysis of the body of the preemptive press releases revealed that, of the preselected words, the most frequently used word was “safety,” which was used a total of 445 times across all of the preemptive press releases. Other common words were “approval” (n = 179), “positive” (n = 119), and “adverse” (n = 98). The NLP-generated heatmap of commonly identified words is included in Figure 2.
Figure 2.
Heatmap of Selected Keywords Included in the Preemptive Press Release.
Overall, 91% of studies with a preemptive press release were funded by a pharmaceutical company, compared to 40% of studies without a preemptive press release. In our multivariable logistic regression model, the following study-level variables had the strongest associations with preemptive press release: funded by the pharmaceutical industry (odds ratio [OR] = 13.22, 95% CI [6.92, 25.22]), publication in Lancet (OR = 3.74, 95% CI [1.80, 7.75]), and publication in NEJM (OR = 3.14, 95% CI [1.53, 6.45]; Table 3). Whether or not a study’s primary outcome was positive was not associated with higher odds of having a preemptive press release; however, most studies with a preemptive press release were positive, which limited statistical power to detect an association.
Table 3.
Multivariable Logistic Regression Results Identifying Factors Associated with Preemptive Press-Release
| Covariate | Reference | OR | CI |
|---|---|---|---|
| Year of publication | |||
| 2016 | 2015 | 1.70 | (0.93, 3.11) |
| 2017 | 2015 | 1.72 | (0.97, 3.05) |
| 2018 | 2015 | 1.45 | (0.79, 2.65) |
| 2019 | 2015 | 0.91 | (0.51, 1.63) |
| Journal | |||
| Lancet | JAMA | 3.74 | (1.80, 7.75) |
| NEJM | JAMA | 3.14 | (1.53, 6.45) |
| Trial sample size | |||
| Large sample (≥ 1000) | Small sample (< 1000) | 1.16 | (0.78, 1.75) |
| Disease area | |||
| Cardiovascular | Non-cardiovascular | 0.64 | (0.37, 1.10) |
| Funding source | |||
| Pharmaceutical funding | Non-pharma | 13.22 | (6.92, 25.22) |
| Primary endpoint result | |||
| Neutral | Negative | 0.44 | (0.11, 1.85) |
| Positive | Negative | 0.72 | (0.18, 2.86) |
| Blinding | |||
| Blinded | Unblinded | 0.86 | (0.59, 1.26) |
Abbreviations: 95% CI, confidence interval; JAMA, Journal of the American Medical Association; NEJM, New England Journal of Medicine; OR, odds ratio
Studies with a preemptive press release had a similar Altmetric score (median 216 [IQR 105, 390]) to those without a preemptive press release (median 229, [IQR 120, 468]). In our multivariable model adjusting for potential confounders, this corresponded to a similar median Altmetric score (median − 15, 95% CI − 33, 12). Studies with a preemptive press release were associated with having a higher number of subsequent citations (median 225, [IQR 101, 428]) than those without a preemptive press release (median 116, [IQR 58, 232]). In our multivariable model adjusting for potential confounders, this corresponded to a median of 22 more citations (95% CI 10, 33) for studies with a preemptive press release compared to those without (Appendix Table 1). While this adjusted difference (i.e., median 22 more citations) is lower than the unadjusted difference of a median of 109 more citations (i.e., 225 vs 116), this commonly occurs when comparing unadjusted estimates to estimates from a multivariable regression model because the latter adjusts for confounding factors while the former does not.
DISCUSSION
In this study of RCTs published in NEJM, JAMA, and Lancet between 2015 and 2019, approximately one in five had a preemptive press release. These preemptive press releases were published an average of > 200 days in advance of the RCTs’ publication in a peer-reviewed journal. RCTs with preemptive press release had higher citation counts. The strongest predictor of whether a study would have a preemptive press release was the funding source, with studies funded by pharmaceutical companies being far more likely to have a preemptive press release. Taken together, these results confirm that preemptive press releases are common, occur many months before the article is published, and are most common for studies funded by a pharmaceutical company.
Preemptive press releases allow for compliance with regulations set out by the Securities and Exchange Committees, as pharmaceutical companies have a fiduciary responsibility to share study results with their investors.12 Preemptive press releases also raise awareness of study results: we were able to quantify the impact of preemptive press release on knowledge dissemination as suggested by the higher citation count we observed. The International Committee of Medical Journal Editors acknowledges the responsibility of journal editors to provide timely access to scientific results, and therefore encourages the use of an embargo system.13 NEJM, JAMA, and Lancet all grant prepublication access with varying embargo times, as a way of facilitating access to the full-text article for journalists and media, prior to publication.14–16 Although this encourages a more responsible and accurate reporting of scientific literature, these embargo times are often short (e.g., 1–7 days prior to publication), and journalists may only publish once the embargo is lifted. As demonstrated by our study results, preemptive press release publication far predates these embargo times.
From the perspective of patients and clinicians, one concern with preemptive press releases is whether they contain enough information to adopt the results into clinical practice prior to access to the full publication. This is primarily relevant for medications that are already available on the market at the time of the press release. The most recent example is the RECOVERY trial, which identified that dexamethasone improved mortality for patients hospitalized with COVID-19 who were hypoxic.17 The researchers released their results via a preemptive press release, but they also provided access to their study protocol and other trial-related details. This is ideal because it provides clinicians with precise details on how the study was designed, its analysis plan, and other details needed to help interpret the results. In the absence of these materials, a press release alone would lack sufficient detail to understand the nuances of the trial (e.g., inclusion criteria, exclusion criteria) that are essential to fully understanding how the trial results should be applied to clinical practice.
Our study also identified there were, on average, over 200 days between the dissemination of a press release and publication in one of three high-impact journals. This aligns with a study of phase 3 clinical trials in oncology, which found that publication of trial results or posting of results to ClinicalTrials.gov typically occurred 300 days after the press release.18 In that particular study, the researchers did not specifically analyze the content of the headline, but other studies have identified a tendency for press releases to present a positive “spin” on study results.3 We observed concordance between a press release having a positive headline and the associated study having a positive primary outcome. Our interpretation was subjective and unblinded, which has the potential to bias our findings. For this reason, we also conducted an analysis using natural language processing. In this analysis, we identified concordance between the trial’s primary outcome and the tone of the press release. Another concern with preemptive releases is that they may lead to selective reporting of results; however, we identified that this was seldom the case. For example, it was rare for the press releases to emphasize a secondary endpoint rather than their primary endpoint. An important limitation of our study, however, is that we only focused on three of the highest-impact clinical journals, and thus the tone and the content of the press releases associated with these publications might not generalize to all clinical journals.
Another important finding of our study was that funding by a pharmaceutical company was overwhelmingly the strongest predictor of studies having a preemptive press release. A prior study, looking at phase 3 trials for oncology medications, showed that the stock prices of a pharmaceutical company are directly related to the results of their RCTs: for the 120 trading days prior to the first press release of trial results, the mean stock price increased by approximately 14% for companies that reported positive trial results and decreased by 1% for companies that reported negative trial results (P = 0.09).19 The authors comment that these findings may be indicative of insider trading.20, 21 One justification for preemptive press releases might be to mitigate the possibility of early leaks, thus preempting insider trading. Because delays in publishing can span many months to over a year depending on the journal, researchers may argue they are aiming to maximize the positive impact they can have on patient outcomes by sharing their results earlier.22 Since 2020, preprint servers (i.e., medRxiv) are widely used for clinical papers; hopefully, in the future, a preprint will accompany the press release.
A strength of our study is that in addition to characterizing the practice of preemptive press release, we were also able to estimate the impact of this practice on knowledge dissemination. We identified a similar Altmetric score (a marker of knowledge dissemination in the lay media) for studies with and without a preemptive press release. In contrast, we identified that having a preemptive press release was associated with a greater number of subsequent citations compared to studies without a preemptive press release. However, in this retrospective study, it is not possible to discern causal relationships. It is possible that studies of greater scientific importance had a preemptive press release rather than the preemptive press release being the reason for the higher citation count.
There were important limitations of our study. First, we restricted our study to articles published in three high-impact general medical journals, and thus our results might not apply to RCTs published in sub-specialty journals or other general medical journals. Second, we focused on articles between 2015 and 2019, and thus our results may not apply to newer randomized trials, especially those related to COVID-19. Many of the largest trials for treatments and vaccines for COVID-19 were initially shared through press release; evaluating the impact of preemptive press releases in the COVID-19 era and beyond will be an important area of future research. Third, it is possible that some press releases may have been missed if they were removed from the internet.
CONCLUSION
Our study identified that approximately one in five RCTs of medications published in high-impact journals between 2015 and 2019 had preemptive press releases that predated the study’s publication by more than 200 days. The strongest predictor for a study to publicize their results in this way was funding from a pharmaceutical company. As preemptive press releases continue to be published, it may be beneficial for authors to be required to publish their article via a preprint server prior to (or concurrently with) publication of the associated press release. Entrenching this practice would allow clinicians and journalists to have access to granular information about the harms and benefits of the intervention as soon as the results are first made public.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
Study concept and design: All authors
Acquisition of data: All authors
Analysis/interpretation of data: All authors
Drafting of the manuscript: Purohit and Fralick
Critical revision of the manuscript: All authors
Statistical analysis: Purohit, Zhu, Fralick
Funding
This study was funded by the Sinai Health Department of Medicine Research Fund.
Data Availability
All data sources used for this study are publicly available.
Declarations:
Conflict of Interest:
MF was a consultant for ProofDx, a start-up company that has created a point-of-care test for COVID-19 using CRISPR. JSR currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST), from the Food and Drug Administration for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164, R01HL144644), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International; in addition, JSR is an expert witness at the request of Relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Woloshin S, Schwartz LM. Press releases: translating research into news. JAMA. 2002;287(21):2856-2858. [DOI] [PubMed]
- 2.Chapman S, Derrick G. Bibliographic analysis of papers and authors published in Tobacco Control 1998-September 2011. Tob Control. 2012;21(2):198-201. [DOI] [PubMed]
- 3.Yavchitz A, Boutron I, Bafeta A, et al. Misrepresentation of randomized controlled trials in press releases and news coverage: a cohort study. PLoS Med. 2012;9(9):e1001308. [DOI] [PMC free article] [PubMed]
- 4.Fralick M, Sacks CA. Publicising trial results before peer review. BMJ. 2019;364:l556. [DOI] [PubMed]
- 5.Landmark NIH study shows intensive blood pressure management may save lives. National Institutes of Health (NIH). Published September 11, 2015. https://www.nih.gov/news-events/news-releases/landmark-nih-study-shows-intensive-blood-pressure-management-may-save-lives.Accessed 6 July 2022.
- 6.SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed]
- 7.Kuriya B, Schneid EC, Bell CM. Quality of pharmaceutical industry press releases based on original research. PLoS ONE. 2008;3(7):e2828. [DOI] [PMC free article] [PubMed]
- 8.Fralick M, Ray M, Fung C, Booth CM, Mallick R, Clemons MJ. Bevacizumab for advanced breast cancer: hope, hype, and hundreds of headlines. Oncologist. 2013;18(11):1174-1179 [DOI] [PMC free article] [PubMed]
- 9.Liu Y, Ott M, Goyal N, et al. RoBERTa: A robustly optimized BERT pretraining approach. arXiv [csCL]. Published online July 26, 2019. 10.48550/ARXIV.1907.11692.
- 10.Yang X, Bian J, Hogan WR, Wu Y. Clinical concept extraction using transformers. J Am Med Inform Assoc. 2020;27(12):1935-1942. [DOI] [PMC free article] [PubMed]
- 11.The donut and Altmetric Attention Score. Altmetric. Published July 9, 2015. https://www.altmetric.com/about-our-data/the-donut-and-score/. Accessed 6 July 2022.
- 12.Goldacre B, Lane S, Mahtani KR, et al. Pharmaceutical companies’ policies on access to trial data, results, and methods: audit study. BMJ. 2017;358:j3334. [DOI] [PubMed]
- 13.Journals and the Media. International Committee of Medical Journal Editors. Published 2023. https://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/journals-and-the-media.html.Accessed 3 April 2023.
- 14.Embargo Policy. New England Journal of Medicine. https://www.nejm.org/author-center/embargo-policy.Accessed 3 April 2023.
- 15.Embargo Policy. JAMA Network. Published 2016. https://media.jamanetwork.com/embargo-policy/. Accessed 3 April 2023.
- 16.Publishing excellence. The Lancet. https://www.thelancet.com/publishing-excellence.Accessed 3 April 2023.
- 17.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed]
- 18.Qunaj L, Jain RH, Atoria CL, Gennarelli RL, Miller JE, Bach PB. Delays in the publication of important clinical trial findings in oncology. JAMA Oncol. 2018;4(7):e180264. [DOI] [PMC free article] [PubMed]
- 19.Rothenstein JM, Tomlinson G, Tannock IF, Detsky AS. Company stock prices before and after public announcements related to oncology drugs. J Natl Cancer Inst. 2011;103(20):1507-1512. [DOI] [PubMed]
- 20.Benowitz S. Big business: when Wall Street and cancer research collide. J Natl Cancer Inst. 2002;94(18):1352-1353. [DOI] [PubMed]
- 21.Herper M. Winners and losers from cancer research’s biggest event. Forbes. Published June 4, 2018. https://www.forbes.com/sites/matthewherper/2018/06/04/winners-and-losers-from-cancer-researchs-biggest-event/.Accessed 6 July 2022.
- 22.Björk BC, Solomon D. The publishing delay in scholarly peer-reviewed journals. J Informetr. 2013;7(4):914-923.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data sources used for this study are publicly available.