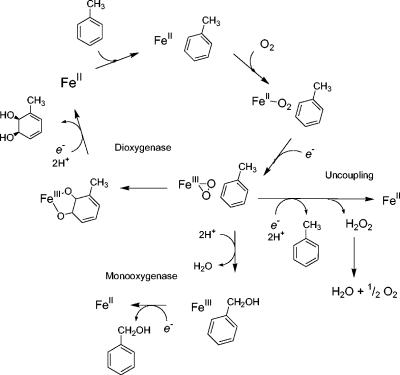
FIG. 5.
Alternative pathways for dioxygenase and monooxygenase activities and uncoupling. Dihydroxylation results from the addition of O2 and two-electron reduction to the peroxo derivative. In the structure of naphthalene dioxygenase with O2 and substrate in the active site (24), the oxygen is bound side-on to the iron and next to the aromatic ring. Addition of both oxygen atoms, with two protons, would lead to addition of two —OH groups. The monooxygenation reactions are concerted reactions in which one oxygen atom is protonated and reduced to H2O and the other oxygen atom is inserted into the C—H bond of the side chain. The uncoupling reaction is shown as a protonation of the Fe-peroxo species, releasing H2O2.