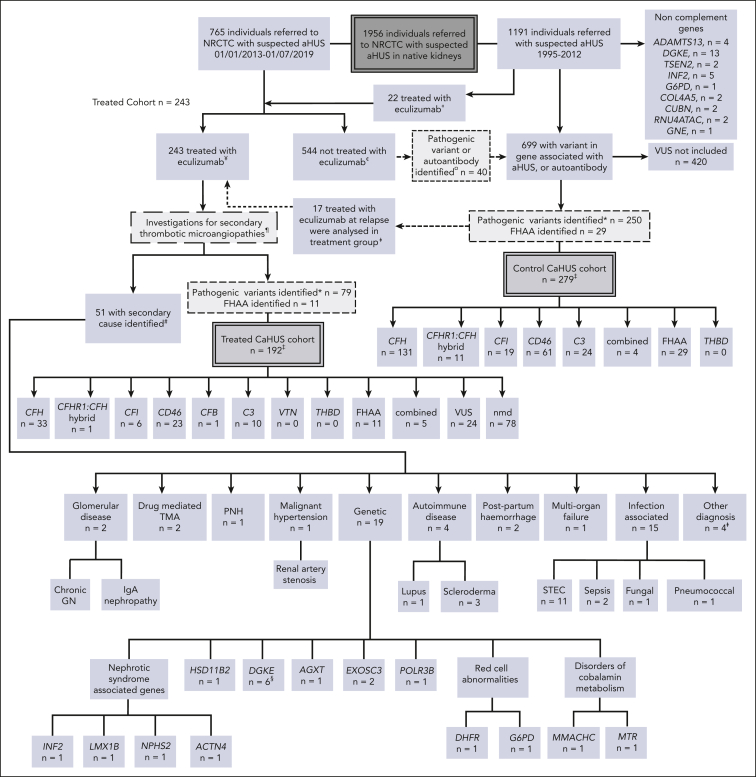
Figure 1.
Patient selection. All individuals referred to the NRCTC with suspected aHUS were considered for study entry. Individuals referred between 1995 and 2012 (before the approval of eculizumab) and those not treated with eculizumab were retrospectively identified. Those with a pathogenic mutation in a gene associated with aHUS or a positive factor H autoantibody were included in the control cohort. Individuals referred to the National Service between 2013 and July 2019 with suspected aHUS who received eculizumab for native kidney disease were prospectively identified and included in the treated cohort. Recipients who had undergone kidney transplantation were excluded. Individuals referred after 2013 but who did not receive eculizumab, were included in the control cohort. THBD and VTN mutations have been reported in aHUS but were not detected in our cohort, and the PLG susceptibility variant c.1481C>T42 was identified in 1 individual who had compound heterozygous DGKE mutations. «Twenty-two individuals were treated with eculizumab between 2010 and 2012, before regulatory approval, either as part of a clinical trial or on compassionate grounds. ¥Excluding individuals treated preemptively during kidney transplantation or posttransplantation. In addition to eculizumab, treatment could have comprised supportive management, renal replacement therapy, and plasma exchange. ¢Management determined by the treating physician could have comprised supportive management, renal replacement therapy, and plasma exchange. ¤Of the 40 individuals, 24 recovered renal function, 15 developed/presented with ESKD, and 1 died. See supplemental Table 1 for genetic and clinical details. ꬸFor survival analysis, 17 individuals were analyzed in the control CaHUS group until the point at which they received eculizumab for relapse and were then analyzed in the treated CaHUS group. CFH, n = 8; CD46, n = 7; C3, n = 1; combined, n = 1. ‡For 2 individuals (nmd) in the treated CaHUS cohort and 13 individuals in the control CaHUS cohort (CFH, n = 5; CFHR1 hybrid, n = 1; CFI, n = 1; CD46, n = 4; C3, n = 1; and FHAA, n = 1), survival data were not available. ¶Thrombotic thrombocytopenic purpura is excluded before treatment with eculizumab: treatment is not commenced if ADAMTS13 <10%. Other secondary TMAs may be identifiable before the initial decision to commence eculizumab based on clinical history or initial laboratory testing: disseminated intravascular coagulation; malignancy-associated TMA; bone marrow transplantation–associated TMA; de novo TMA after solid organ transplantation; and drug-induced TMA. Some secondary TMAs may not be identified until further genetic or serological tests or kidney biopsies are available: Shiga toxin HUS (STEC-HUS); pneumococcal HUS; HIV; cobalamin C deficiency TMA; glomerular disease–associated TMA; and autoimmune disease–associated TMA. #No complement mutations and no FHAA were identified in any of those with a secondary cause identified. §PLG screened only for the susceptibility variant c.1481C>T rs4252128; identified in 1 individual (with compound heterozygous DGKE mutations). ꬸOther diagnoses, n = 4: renal biopsy specimen showed severe chronic damage, no TMA detected with a renal biopsy specimen; renal biopsy specimen showed acute tubular necrosis; no TMA detected with a renal biopsy specimen; died before definitive diagnosis made. ∗Analysis of variants in July 2020 classified those with definitive evidence of functional significance as pathogenic and those without as VUS. GN, glomerulonephritis; IgA, immunoglobulin A; PNH, paroxysmal nocturnal hemoglobinuria.