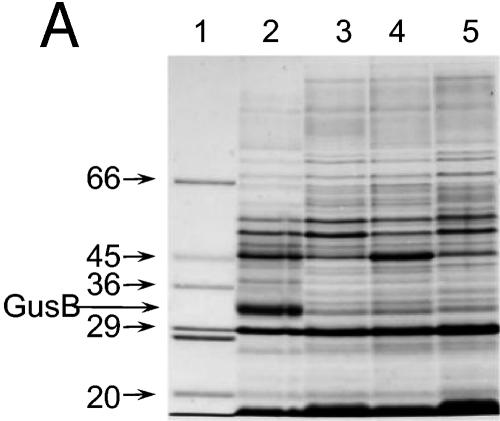
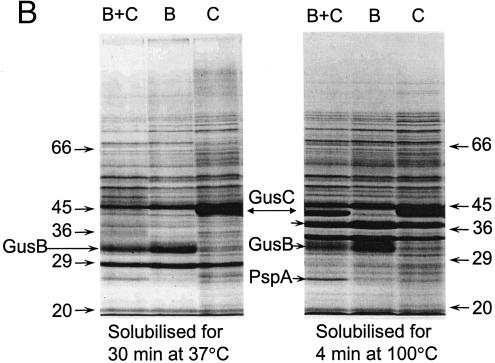
FIG. 3.
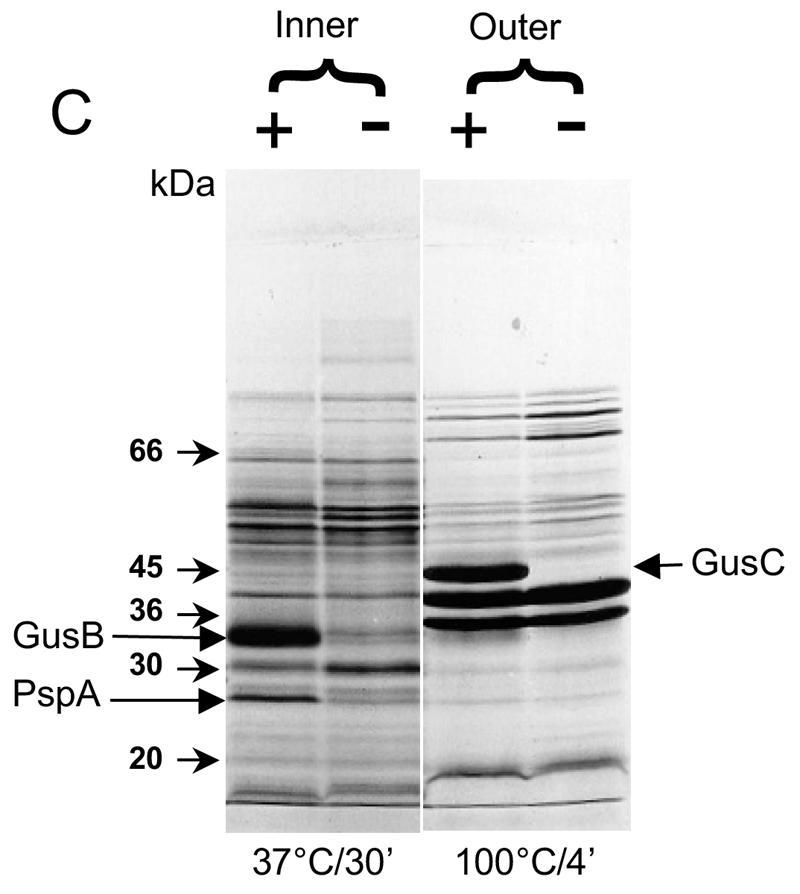
Appearance of putative GusB and GusC proteins in membrane preparations. (A) Total membrane proteins prepared from E. coli strain NO2947 harboring plasmids as indicated below were incubated in solubilization buffer at 37°C for 30 min; 45 μg of proteins was loaded onto each track of the SDS-12% bisacrylamide gel. Lane 1, protein size markers (in kilodaltons); lane 2, plasmid pWJL24 tac-gusBC induced; lane 3, pWJL24 tac-gusBC uninduced; lane 4, pTTQ18 induced control; lane 5, no plasmid in host cell control. (B) After solubilization conditions as shown, total membrane protein (45 μg) was loaded onto each track of the SDS-12% bisacrylamide gel. Lane B+C, pWJL24 tac-gusBCCE1 induced; lane B, pWJL26 tac-gusB CE1 induced; lane C, pWJL31 tac-gusCCE1 induced. Proteins in lanes 4, 5, and 6 were as same as those in lane 1, 2, and 3, respectively. (C) GusB occurs in the inner membrane and GusC in the outer membrane. Membrane fractionation was carried out by sucrose gradient and ultracentrifugationafter cell lysis by French press (20,000 lb/in2). Inner, inner membrane proteins solubilized at 37°C for 30 min; Outer, outer membrane proteins solubilized at 100°C for 4 min. +, proteins from NO2947(pWJL24) with induction of = tac-gusBCCE1; −, proteins from NO2947(pWJL24) without induction of tac-gusBCCE1.