Abstract
Iron imbalance in the brain negatively affects brain function. With aging, iron levels increase in the brain and contribute to brain damage and neurological disorders. Changes in the cerebral vasculature with aging may enhance iron entry into the brain parenchyma, leading to iron overload and its deleterious consequences. Endothelial senescence has emerged as an important contributor to age‐related changes in the cerebral vasculature. Evidence indicates that iron overload may induce senescence in cultured cell lines. Importantly, cells derived from female human and mice generally show enhanced senescence‐associated phenotype, compared with males. Thus, we hypothesize that cerebral endothelial cells (CEC) derived from aged female mice are more susceptible to iron‐induced senescence, compared with CEC from aged males. We found that aged female mice, but not males, showed cognitive deficits when chronically treated with ferric citrate (FC), and their brains and the brain vasculature showed senescence‐associated phenotype. We also found that primary culture of CEC derived from aged female mice, but not male‐derived CEC, exhibited senescence‐associated phenotype when treated with FC. We identified that the transmembrane receptor Robo4 was downregulated in the brain vasculature and in cultured primary CEC derived from aged female mice, compared with those from male mice. We discovered that Robo4 downregulation contributed to enhanced vulnerability to FC‐induced senescence. Thus, our study identifies Robo4 downregulation as a driver of senescence induced by iron overload in primary culture of CEC and a potential risk factor of brain vasculature impairment and brain dysfunction.
Keywords: cellular senescence, cerebral endothelial cells, iron overload, molecular biology of aging, sex characteristics
The brain function of aged female mice is more vulnerable to chronic administration of iron supplement ferric citrate (FC), compared with aged male mice. Senescence‐associated phenotype is exacerbated by FC in cultured primary cerebral endothelial cells (CEC) isolated from aged female mice, but not in CEC from aged male mice. Robo4 is downregulated in the cerebrovasculature of aged female mice, versus males, and CEC from aged male mice become more susceptible to FC after Robo4 downregulation.

Abbreviations
- BBB
blood brain barrier
- CEC
cerebral endothelial cells
- FC
ferric citrate
- m/o
months old
- SA
senescence associated
1. INTRODUCTION
The proper balance of iron uptake and release is critical for brain function (Hare et al., 2013). On one hand, iron deficiency negatively affects both the brain connectome (Algarin et al., 2017; Li et al., 2021) and hippocampal plasticity (Nelissen et al., 2017). Iron deficiency is associated with fibromyalgia (Yao et al., 2021) and anxiety (Li et al., 2011). On the other hand, with aging, iron levels increase in the brain (Sato et al., 2022), which contributes to oxidative stress, cell death, and brain damage (Yan et al., 2021). While iron deficiency has been extensively studied, there has been less investigation of the effects of iron overload. Importantly, elevated brain iron may contribute to Alzheimer's and Parkinson's diseases, and other neurological disorders (Ndayisaba et al., 2019).
Cerebral endothelial cells (CEC) are the major component of the most restrictive barrier between the circulating blood and the brain. Thus, any deleterious changes in CEC may initiate detrimental consequences for the entire brain (Marques et al., 2013). Cell senescence is a non‐proliferative state characterized by accumulation of DNA damage and the development of a pro‐inflammatory secretory phenotype that negatively affects the neighboring cells and tissues (Del Valle et al., 2009; Graves & Baker, 2020; Pelegrí et al., 2007; Yamazaki et al., 2016; Yang, Sun, et al., 2017; Yang, Yang, et al., 2017). Accumulation of senescent CEC occurs with aging (Kiss et al., 2020) and may alter the cerebrovasculature by dysregulating cerebral blood flow, increasing blood brain barrier (BBB) permeability, and changing the cerebrovasculature architecture (Del Valle et al., 2009; Yang, Sun, et al., 2017; Yang, Yang, et al., 2017).
Iron access to the brain occurs through the BBB and the blood cerebrospinal fluid barrier via transferrin‐mediated endocytosis. Iron overload and iron deposition are associated with cell senescence in cultured cell lines (Angelova & Brown, 2018; Killilea et al., 2003; Masaldan et al., 2018). However, whether iron overload induces senescence in primary culture of CEC and in the brain vasculature of aged mice have not been studied.
Ferric citrate (FC) is a common food additive and it is used as an oral iron supplement for the treatment of iron deficiency anemia in chronic kidney disease patients. FC treatment is very effective in increasing both hemoglobin and iron indexes in chronic kidney disease patients (Block et al., 2015; Fishbane et al., 2017; Lee et al., 2022). However, preclinical studies have found that FC can increase the risk of aluminum toxicity (Gupta, 2014) and induce neurodegeneration, and motor and cognitive deficits (Huang et al., 2019). As anemia is commonly found in the elderly population (≥65 years old) (Halawi et al., 2017), and this group has highest use of dietary supplements and prescription medications (Gahche et al., 2017), and unfortunately, as self‐medication is a frequent practice among the elderly population (Oliveira et al., 2018), the study of the effects of iron overload in aging models is clinically relevant.
In this study, we used aged (18–20 months old, m/o) mice of both sexes treated with FC (Gupta, 2014). We found that the brain function and the cerebrovasculature in aged female mice were more vulnerable to iron overload than in aged male mice. In in vitro experiments, we found that cultured primary CEC derived from aged female mice were more susceptible to become senescent by FC, compared with male CEC. RNA sequencing (RNA‐seq) revealed that the gene that codes for the endothelial transmembrane protein Robo4 was downregulated in cultured CEC and in brain microvessel isolated from aged female mice, compared with those from male mice. Importantly, we found that Robo4 downregulation sensitized male mouse‐derived CEC to become senescent by FC.
2. EXPERIMENTAL PROCEDURES
2.1. Animals and ethics statement and treatment
C57BL/6J male and female mice (18–20 m/o) from National Institute of Aging were housed in the animal facilities at the University of Texas McGovern Medical School. The experiments were conducted following the protocol approved by the Center for Laboratory Medicine and Care (protocol number AWC‐21‐0084) at the University of Texas McGovern Medical School. Mice were maintained in an environment with constant temperature and humidity on a 12 h light/12 h dark schedule, and with ad libitum access to water and mouse lab pellets. Researchers and veterinarians maintained mice with no distress, pain, or injury.
Mice were treated with 200 μL of 5% FC (in saline solution), or with 200 μL of physiological saline solution as control, by oral gavage alternatively 3 days per week for 6 weeks. Each cage contained FC‐ and vehicle‐treated mice. During the duration of the treatment, mice were maintained in the same conditions of temperature, humidity, light, and food/water as described above. All experiments were performed by investigators blinded to sex and treatment.
2.2. Reagents
Antibodies against actin (3700) were obtained from Cell Signaling. Antibodies against γH2AX (ab11174) were from Abcam. FC was from Sigma (F3388). Hoechst dye (#SC‐394039) and anti‐ICAM1 (#sc‐107) were from Santa Cruz Biotechnology. ON‐TARGETplus mouse Robo4 SMARTPool siRNA (L‐050278‐01) and ON‐TARGETplus mouse non‐targeting SMARTPool (D‐001810‐10) were from Horizon Discovery.
2.3. Isolation of cerebral endothelial cells from adult mouse brains and culture
Primary CEC were isolated from adult 18–20 m/o old C57BL/6J male and female mice using the Adult Brain Dissociation Kit (MACS Miltenyi Biotec, #130‐107‐677) as previously described (Noh et al., 2022). Briefly, brain tissue was mechanically and chemically homogenized, and filtered through 70 μm filters. Debris was separated by centrifugation, and red blood cells were removed. Then, the cell suspension was plated with Complete Mouse Endothelial Cell Medium/w Kit (Cell Biologics, #M1168) supplemented with puromycin (4 μg/mL) and maintained for 48 h to selectively maintain CEC in culture. After 48 h, medium was replaced with fresh complete medium. Complete medium contains 5% of fetal bovine serum, 0.1% VEGF, 0.1% ECGS, 0.1% heparin, 0.1% hydrocortisone, and 1% Antibiotic‐Antimycotic solution. For treatments, cultured CEC were treated with FC (50 or 150 μM) or a vehicle (H2O) in a final volume of 0.8 mL/cm2 for 7 days. Medium was not replaced during the treatments. For experiments with siRNA, cultured CEC were transfected with DharmaFECT (Horizon Discovery) and a total of 5 nM of siRNA against mouse Robo4 (Horizon), or non‐target siRNA as control.
2.4. Isolation of cerebral microvessels from adult mice
Isolation of microvessel fractions from adult mice was previously described (Noh et al., 2022). After obtaining the pellets with microvessels, the pellets were processed to isolate RNA and further perform qPCR or fixed and stained with antibodies.
2.5. Gene expression by qPCR
Cultured CEC or microvessel fractions were collected and processed following the manufacturer's instructions of the RNeasy Mini Kit (Qiagen, #74104) to isolate total RNA. RNA was reverse transcribed with the iScript Reverse Transcription SuperMix (Bio‐Rad, #1708840). cDNAs were then used for RNA‐Seq analyses (Qiagen) or used to analyze the gene expression by qPCR.
For qPCR analysis, cDNA was diluted in iTaq Universal SYBR® Green Supermix (Bio‐Rad, #1725121) and run using a Bio‐Rad CFX384 Touch device (95°C for 3 m, and 40 cycles of 95°C for 10 s, and 55°C for 30 s).
For RNA‐Seq analysis, log10 p‐value >2 was established as a variable to select the most significant differential expressed genes, and log2 fold change < −1.5 and >1.5 as a variable to select the most downregulated and upregulated differential expressed genes, respectively.
2.5.1. Sequences of primers
Fw mouse Gapdh 5′‐CAAGGTCATCCATGACAACTTTG‐3′; Rv mouse Gapdh 5′‐GTCCACCACCCTGTTGCTGTAG‐3′. Fw mouse Robo4 5′‐GTCATTGCCAGTAGTGCTGTCC‐3′; Rv mouse Robo4 5′‐AATGGCGTCCTCGCTGGTGTAT‐3′. Fw mouse p16INK4a (Cdkn2a) 5′‐TGTTGAGGCTAGAGAGGATCTTG‐3′; Rv mouse p16 5′‐CGAATCTGCACCGTAGTTGAGC‐3′. Fw mouse p21CIP1 (Cdkn1a) 5′‐TCGCTGTCTTGCACTCTGGTGT‐3′; Rv mouse p21 5′‐CCAATCTGCGCTTGGAGTGATAG‐3′. Fw mouse IL6 5′‐TACCACTTCACAAGTCGGAGGC‐3′; Rv mouse IL6 5′‐CTGCAAGTGCATCATCGTTGTTC‐3′. The relative expression of the gene of interest was calculated with the double delta Ct method related to the relative expression of Gapdh.
2.6. Immunocytochemistry
Cultured CEC and microvessels were fixed with 4% paraformaldehyde for 10 m at RT, and then permeabilized with 0.1% Triton‐X100 for 10 m at RT. Samples were blocked with 5% bovine serum albumin overnight at 4°C, and then incubated with a solution of primary antibodies in the blocking solution overnight at 4°C. Samples were incubated with Alexa Fluor®‐conjugated secondary antibodies (Abcam) for 1 h at room temperature, and with the Hoechst dye to stain nuclei.
Images from 5 to 10 microscopic fields (x20 or x40 objectives) were taken using the same exposure time and light intensity. We established threshold limits for each marker for quantitative analyses. The mean of fluorescence intensities of the GFP‐LC3‐RFP reporter and the standard deviation of γH2AX in each region of interest was quantified with ImageJ software. The fluorescence intensities from the background of each picture were subtracted from the correspondent values of the region of interest in the same image.
Images were taken with the EVOS FL 2 Auto Imaging System (Thermo Fisher Scientific).
2.7. Western blotting
Brain tissues from experimental mice were processed for electrophoresis and western blotting as previously described (Noh et al., 2022).
2.8. Immunohistochemistry
Brains from perfused and decapitated mice were fixed by immersion in 4% paraformaldehyde overnight at 4°C, and then dehydrated with 30% sucrose for 2 days at 4°C. Brains were sliced (24 μm thickness) with a freezing microtome, and sections were stored with an anti‐freezing solution at −20°C until needed. For Prussian blue staining, brain slices on glass slides were rehydrated with water and incubated with 20% hydrochloric acid–10% potassium ferrocyanide (1:1) solution for 20 m. After washing in distilled water, sections were counterstained with Nuclear fast red for 5 m. Then, slices were rinsed in distilled water and dehydrated with 95% and 100% ethanol and xylene. 20x magnification images were analyzed with Fiji‐ImageJ software to determine the number of iron deposits per area (mm2). Color channels were separated using the vector FastRed FastBlue DAB in the Colour deconvolution command. Then, the same threshold was applied for the blue channel of all images to identify the Prussian blue‐positive particles.
For γH2AX staining, brain slices were rehydrated with Tris‐buffered saline. Slices were incubated with 3% H2O2 for 30 m, blocked with 2% Donkey serum/PBS for another 30 m and then incubated with anti‐γH2AX (1:3000) overnight at 4°C. Then, samples were incubated with anti‐rabbit 1 h and developed with the 3,3′‐diaminobenzidine substrate kit (Vector laboratories, SK‐4100). Samples were washed and dehydrated with 95% and 100% ethanol and xylene. Color channels of 20x magnification images were separated using the vector H DAB in the Colour deconvolution command. Then, the same threshold was applied for the brown channel of all images to identify the γH2AX‐positive particles.
All images from immunohistochemistry studies were obtained on a Keyence BZ‐X810 microscope.
2.9. Measurement of iron content
Brain tissues and blood cell pellets were homogenized with 800 μL of 1 mmol/L HCl–10% trichloroacetic acid solution, and incubated for 1 h at 50°C with shaking. Lysates were centrifuged at 15,000 g for 15 m at RT, and the supernatants were collected. Supernatant (90 μL) was mixed with 30 μL of ascorbic acid (20 mg/mL), and then added 20 μL of a ferrozine solution (0.85% w/v ferrozine in hydroxylamine hydrochloride). Samples were incubated for 30 m at RT and the absorbance was measure at 560 nm. We used Iron Standard (Sigma, #02583) for the standard curve.
2.10. Flow cytometry and ex vivo SA‐β‐gal‐activity assay
Mice (18–20 m/o) were perfused with cold PBS, and the left hemisphere was isolated for single cell processing as described in (Ritzel et al., 2022). Briefly, brains were placed in RPMI 1640 (Invitrogen, #22400105) medium and mechanically and enzymatically digested for 45 m at 37°C on a shaking incubator. The cell suspension was filtered through a 70‐μm filter, and cells were then incubated with TruStain FcX Block (Biolegend, #101320), for 10 m on ice, and stained with CD45‐eF450 (eBioscience, #48–0451‐82), CD31‐PECy7 (Biolegend, #102444), and Tie2‐APC (Biolegend, #124010). The fixable viability dye Zombie Aqua was used for live/dead discrimination (Biolegend, #423102). Cells were then washed in FACS buffer, fixed in 2% paraformaldehyde for 8 m, and washed once more prior to adding 500 μL FACS buffer. Senescence associated (SA) β‐Galactosidase Activity Assay Kit was performed according to the manufacturer's instructions (Cell Signaling Technologies, #35302S).
Data were acquired on a Beckman Coulter CytoFLEX LX cytometer using CytExpert v2.5 (Beckman Coulter) and analyzed using FlowJo (Treestar Inc.). Cells were first gated using a splenocyte reference (SSC‐A vs. FSC‐A). Singlets were gated (FSC‐H vs. FSC‐W), and live cells were gated based on Zombie Aqua exclusion (SSC‐A vs. Zombie Aqua‐Bv510). Brain endothelial cells were identified as the CD45−CD31+Tie2+ population. A tissue‐ and cell type‐matched fluorescence minus one control was used to determine gating for SA‐β‐Gal‐activity.
2.11. Scratch assay
Cultured CEC 100% confluent were scratched with a 200 μL tip. Cells were washed with PBS, and medium with the corresponding treatments was added. The plate was placed in the Incucyte Live‐Cell Analysis System (Sartorius), and cells were imaged every 8 h for 48 h. Distance between edges was measured at five different intervals per image using the ImageJ software. The percentage of the wound closure per time point was calculated relative to the first image taken for each well.
2.12. Behavior
Y‐maze: a mouse was placed in the interjection of the three arms of a Y‐shaped structure (39.5 × 8.5 × 13 cm), and allowed it to move freely through the maze during a 5 m session. Movements were video recorded, and a blinded investigator to the experimental groups analyzed the number of arm entries. The percentage of spontaneous alternation was calculated as [(number of alternations)/(total arm entries − 2)] × 100.
Open Field: a mouse was placed in a squared arena (40 cm per side) and allowed it to explore for 20 m. Movements were video recorded and velocity, distance moved, time spent in the center and borders of the arena, and the frequency of visits to these areas were automatically calculated by the Ethovision XT software.
Novel object recognition: 24 h after the Open Field test, two identical objects were presented to a mouse in the arena, and the mouse was allowed to explore the objects for 10 m. Twenty‐four hours later, one of these objects was replaced with a novel object, and again, the mouse was allowed to explore the novel and the familiar objects for 10 m. We evaluated the differences in the exploration time with novel and familiar objects by calculating the recognition index as [(time with novel object)/(time with novel object + time with familiar object)].
Fear conditioning test: A mouse was placed in an arena (novel environment, 30 cm × 20 cm) in which does not enter light from the room, only a white light installed inside the arena (neutral stimulus). The mouse was allowed to explore for 2 m. 1 h later, the animal was again placed in the arena with the same light and allowed to explore other 2 m, time that was followed by a 1 mA electric foot shock (aversive stimulus) for 2 s. Twenty‐four hours after the aversive stimulus, the animal was returned to the arena and exposed to the light without shock. The percentage of inactive time was recorded.
2.13. Statistics
All analyses were performed using GraphPad Prism software (v.7). We used Student's t test to compare means from two independent groups, and two‐way ANOVA to compare groups with two variables, such as sex and treatment (Table S1). We used Tukey's test for multiple comparisons. A value of p < 0.05 was considered significantly different. Bar graphs represent mean ± SEM.
3. RESULTS
3.1. Cognitive function was more vulnerable to iron overload in aged female mice than in aged male mice
We measured body weight in 18–20 m/o mice of both sexes treated with FC, and found no significant differences between groups (Figure 1a,b). We tested mice for motor skills and anxiety‐like behavior by Open Field test, and found that treatment did not have significant effect. However, sex had a significant effect on velocity (Figure 1c) and distance moved (Figure 1d), being female mice what showed higher velocity and moved longer distances, compared with males. Aged female mice spent less time not moving than aged male (Figure 1e). Furthermore, we did not find differences between sexes or treatment in time spent either in the center of the arena (Figure 1f) or in borders (Figure 1h). Importantly, sex had a significant effect on the number of visits to borders, being female mice what visited this area more often than males (Figure 1i). Post hoc test did not detect significant differences between the four groups in the variables mentioned above.
FIGURE 1.
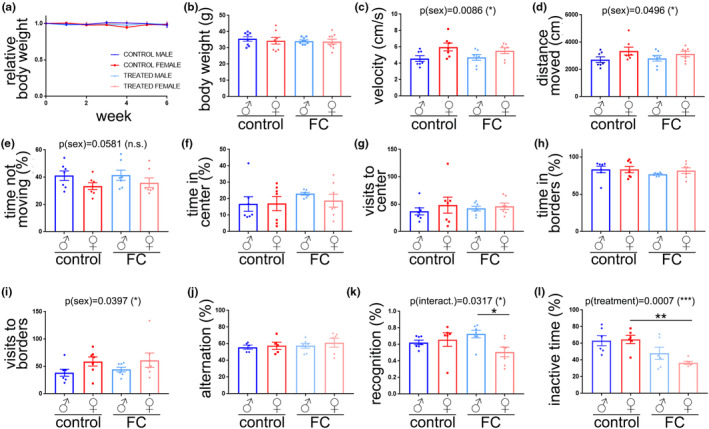
Iron overload impaired recognition and conditioned memory in aged female mice. (a) Relative body weight in mice from FC‐treated and control groups. (b) Absolute body weight 6 weeks after the initiation of the treatments. (c–i) Open field: velocity (c), distance moved (d), percentage of time not moving (e), percentage of time spent in the center (f), number of visits to the center (g), percentage of time spent in the borders (h), and number of visits to the borders (i). (j) Percentage of alternation. (k) Novel object recognition index. (l) Percentage of freezing time. All data are mean ± SEM with n = 6–8. Two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05, **p < 0.01.
We tested spatial memory, recognition memory, and conditioning memory with the Y‐maze, novel object recognition, and fear conditioning tests, respectively. There were no differences in percentage of alternation (Figure 1j). However, we found that the recognition memory was significantly affected by an interaction between treatment and sex (Figure 1k), and that female mice treated with FC showed a significant reduction in the recognition index percentage, compared with FC‐treated males. We did not find significant differences in exploration time with familiar and novel objects between sexes (Figure S1). The conditioning memory was significantly reduced by the treatment with FC (Figure 1l), and FC‐treated female mice showed reduced freezing time compared with control females. Interestingly, there were no significant changes in freezing time between treated and control males. Our data indicate that recognition memory and conditioning memory are more importantly altered by chronic administration of FC in aged female mice, compared with aged males.
As iron can accumulate in the form of iron deposits in the brain with aging, and iron deposition has been associated with brain dysfunction (del C Valdés Hernández et al., 2015), we determined whether iron overdose contributes to iron deposition in the brain in a sex‐dependent manner. Brain samples were stained with Prussian blue to visualize iron deposits. These iron deposits were found in the hippocampus, thalamus, cortex, and striatum (Figure S2). FC treatment had a significant effect on brain iron deposition (Figure 2a,b). The number of iron deposits increased in the brains of female mice treated with FC, compared with control females. However, no significant changes were observed between groups of aged male mice. Iron content in blood cells was not different between groups (Figure 2c); however, FC treatment enhanced the levels of iron in the brain of aged mice in both sexes (Figure 2d).
FIGURE 2.
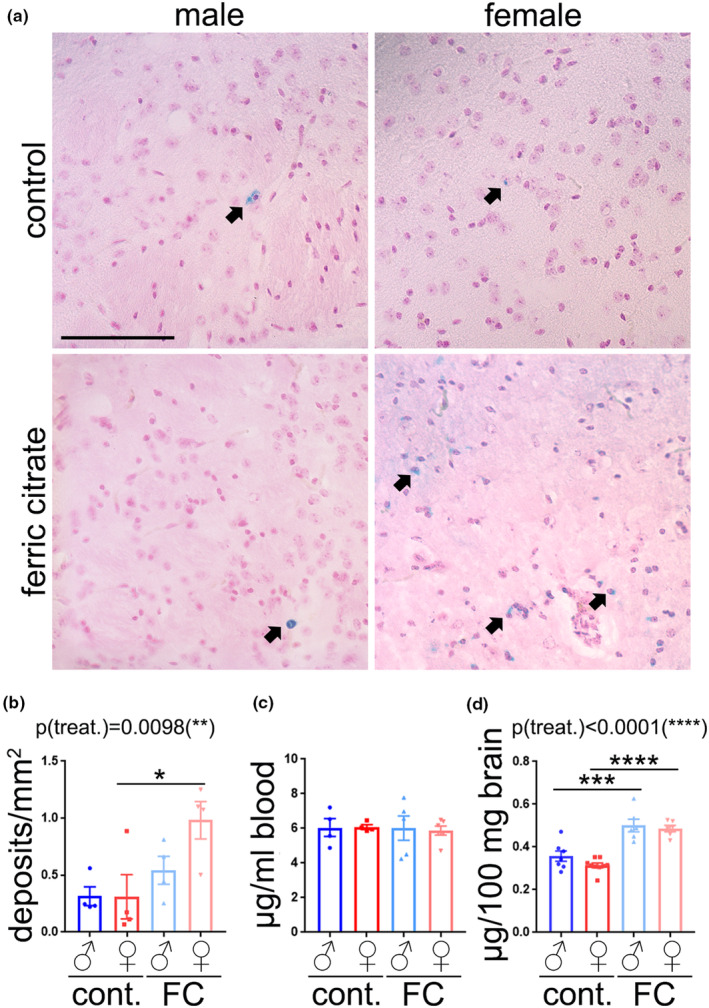
FC treatment enhanced iron deposition in brains of aged female mice, but not in male mouse brains. (a) Representative images of brain sections (cortex region) from aged male and female mice treated with FC or a vehicle stained with Prussian blue (blue) and Nuclear Fast Red (pink). Black arrows depict discrete iron deposits. Scale bar, 100 μm. (b) Number of Prussian blue‐positive deposits per area from (a). Six brain sections per mouse were stained and quantified. Data are mean ± SEM with n = 4. Two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05. (c) Iron content in blood cells of mice of the experimental groups. (d) Iron content in the brain lysates of the experimental groups. Data are mean ± SEM with n = 7–8. ***p < 0.001, ****p < 0.0001. Data are mean ± SEM with n = 4–6.
3.2. Iron overload negatively affected the brain vasculature of aged female mice
Given that iron overload can induce senescence in different cell types in culture (Angelova & Brown, 2018; Cozzi et al., 2019; Curtis et al., 2001; Yang, Sun, et al., 2017; Yang, Yang, et al., 2017; Yuan et al., 2021), and that senescence in the brain has been associated with cognitive deficits (Graves & Baker, 2020; Lin et al., 2021; Sikora et al., 2021), we hypothesized that FC‐induced cognitive dysfunction in females is caused by enhanced senescence in the brain.
Autophagy impairment is one of the features associated with cell senescence (Cayo et al., 2021; Rajendran et al., 2019; Xu et al., 2020). Thus, we analyzed the levels of several autophagy markers in mouse brain lysates (Figure S3a). Treatment had a significant effect on Beclin1 (Figure S3b), Atg7 (Figure S3c), p62 (Figure S3e), Lamp1 (Figure S3f), and LC3‐II (Figure S3d). p62 was significantly affected by sex. FC treatment increased p62 levels in both sexes, and only female mice treated with FC showed significant increases in Lamp1 compared with control female, but no changes were observed in male mice with FC treatment. Altogether, these data suggest that FC stimulates the initial stages of autophagy, but it negatively interferes in the later stages of autophagy, an effect that was more notable in aged female mice than in males.
FIGURE 3.
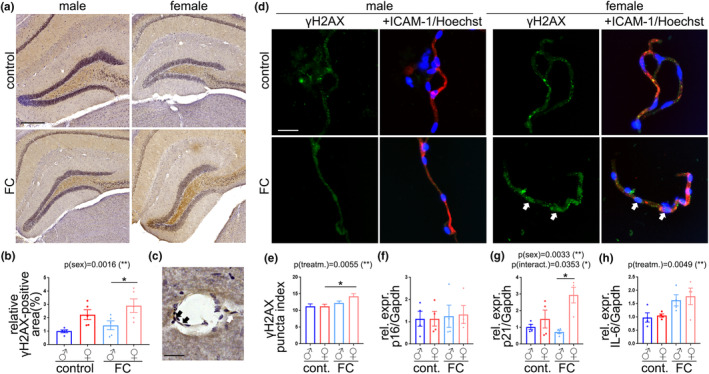
The brain vasculature of aged female mice was more vulnerable to FC than that of aged male mice. (a) Representative images of the hippocampi of mice from control or FC groups stained with anti‐γH2AX (brown) and Hematoxylin (purple). Scale bar, 250 μm. (b) Relative area positive to γH2AX from (a). Data are mean ± SEM with n = 5. (c) Representative image of a cerebral vessel positive to γH2AX (black arrows). Scale bar, 25 μM. (d) Representative images of cerebral microvessels from mice from control or FC groups stained with anti‐γH2AX (green) anti‐ICAM‐1(red) and Hoechst (blue). Scale bar, 25 μM. (e) Puncta index of γH2AX from (d). Data are mean ± SEM pooled from >100 microvessels from four mice/group. (f–h) Gene expression of p16 (f), p21 (g), and IL6 (h) relative to Gapdh. Data are mean ± SEM pooled from four mice/group. All experiments were analyzed by two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05.
DNA damage is the most important contributor to cell senescence (Yousefzadeh et al., 2021). Thus, we determined if chronic administration of FC induces DNA damage in the brains of aged mice of both sexes. Brain samples were stained with antibodies against γH2AX, commonly used as a marker of double strand DNA damage, the most deleterious form of DNA damage (He et al., 2016) (Figure 3a). We analyzed the hippocampus as recognition and conditioning memories are regulated by this brain area (Broadbent et al., 2004; Kim & Cho, 2020). The percentage of brain area positive to γH2AX was more pronounced in female mice than in male in control condition, and, importantly, FC significantly exacerbated differences between sexes (Figure 3b).
Further examination of the brain images revealed that the blood vessels can be positive to γH2AX staining (Figure 3c, arrows). To further investigate the effects of FC particularly on cerebral microvessels, we isolated the microvessel fractions from the mouse brains. We measured the puncta index of γH2AX, which represents the distribution of γH2AX as discrete puncta (DNA lesions) when the index is higher (Moruno‐Manchon et al., 2017). The puncta index of γH2AX was significantly increased in the microvessel fraction of aged female mice treated with FC, compared with control female, whereas we did not find significant differences between groups in the microvessels from male mice (Figure 3d,e). We also analyzed the relative gene expression of p16Ink4a (Cdkn2a), p21Cip1 (Cdkn1a), and IL6 from the microvessel fractions derived from female and males treated with FC or a vehicle (Figure 3f–h). Sex had a significant effect on p21Cip1 expression (Figure 3g), and treatment had a significant effect on IL6 (Figure 3h). We also found an interaction between treatment and sex in p21Cip1 expression as well (Figure 3g). Microvessel fractions of aged female mice treated with FC showed enhanced expression of p21Cip1, compared to the microvessel fractions from treated male mice.
Given that endothelial senescence can negatively affect brain vasculature integrity, we wondered if FC induces vasculature impairment. We analyzed the levels of two common tight junction proteins: ZO‐1 and claudin‐5 (Figure S4). We did not find significant differences in ZO‐1 levels between groups (Figure S4b). However, sex had a significant effect on the levels of claudin‐5 (Figure S4c), and claudin‐5 was significantly increased in the brains of female mice treated with FC, compared with treated male mice.
FIGURE 4.
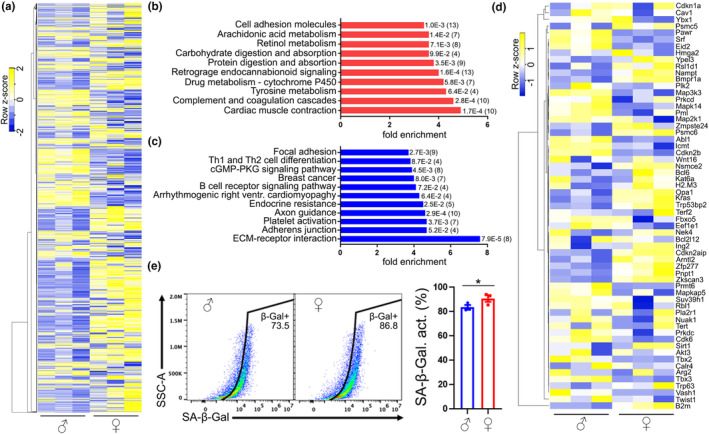
The transcriptome of cultured CEC isolated from aged mice showed sex differences. (a) Heatmap of the 2500 most expressed genes in cultured CEC isolated from 18 to 20 m/o male and female mice. (b,c) Representation of the most enriched KEGG pathways containing the most significant upregulated (b) and downregulated (c) genes in aged female‐derived CEC, compared with aged male‐derived CEC. Each KEGG pathway shows a p‐value and, in brackets, the number of genes in each group. (d) Heatmap of a SA genes in cultured CEC isolated from 18 to 20 m/o male and female mice. (e) Dot plot and bar graph of the percentage of SA‐β‐Galactosidase activity in CD31‐positive cells isolated from aged male and female mice. Data are mean ± SEM pooled from three mice/group. Student's t test, *p < 0.05.
This evidence suggests that the brain vasculature of aged female mice is more vulnerable to FC than that of aged males.
3.3. Cultured primary CEC derived from aged mice showed sex differences in SA phenotype
We then sought to identify the molecular mechanism/s that induce sex‐biased senescence in the brain vasculature of aged mice treated with FC. We used an in vitro model of cultured CEC derived from 18 to 20 m/o mice. We previously showed that our primary culture of CEC conserves important characteristics of the cerebrovasculature in mice, and that it is relevant to study mechanisms related to aging (Noh et al., 2022).
First, we analyzed the transcriptome profiles of these cells by RNA‐Seq. CEC isolated from aged male and female mice showed robust differences (Figure 4a). We identified 1202 and 315 differential expressed genes upregulated and downregulated, respectively, in CEC derived from aged female mice, compared with CEC from aged male mice. We found that genes involved in cardiac muscle contraction, complement and coagulation cascades, and in metabolism were the most significant upregulated genes in aged female mouse‐derived CEC, compared with aged male CEC (Figure 4b). Genes involved in extracellular matrix‐receptor interactions, adherens junction and focal adhesion, and platelet activation were significantly downregulated in aged female‐derived CEC compared with male CEC (Figure 4c).
Next, we aimed to determine if there are sex differences in SA phenotype. In a heat map of genes associated with cell senescence (Figure 4d), we found that female‐derived CEC, compared with male‐derived CEC, showed upregulated expression of multiple genes that promote senescence, such as Nampt (Ma et al., 2017), Opa1 (Tezze et al., 2017), KRas (Lee & Bar‐Sagi, 2010), Zfp277 (Negishi et al., 2010), and Zkscan3 (Hu et al., 2020). In addition, female CEC also showed downregulated expression of genes associated with senescence prevention, such as Srf (Ding et al., 2001), Map3k3 (Zhou et al., 2022), Abl (Zhang et al., 2013), Prmt6 (Stein et al., 2012), Cdk6 (Ruas et al., 2007), and Tbx3 (Kumar et al., 2014). Furthermore, we found by flow cytometry that a higher percentage of cells positive to the endothelial marker CD31 isolated from aged female mice were positive to SA‐β‐Galactosidase, compared with CD31‐positive cells from aged male mice (Figure 4e). This supports our hypothesis that endothelial cells derived from aged female mice are more susceptible to enter into a senescent state, compared with endothelial cells derived from aged male mice.
3.4. Cultured primary CEC derived from aged female mice were more vulnerable to FC than CEC from aged male mice
One of the characteristics of senescent endothelial cells is the inability to migrate properly (Liu et al., 2022; Yang et al., 2018). To analyze migration, we performed a scratch assay in CEC derived from aged male and female mice treated with FC (50, or 150 μM) or a vehicle (Figure 5a–c). Sex, treatment, and their interaction had significant effects on wound closure (Figure 5d), which was significantly reduced in aged female‐derived CEC treated with 150 μM FC, compared with male CEC at the same conditions.
FIGURE 5.
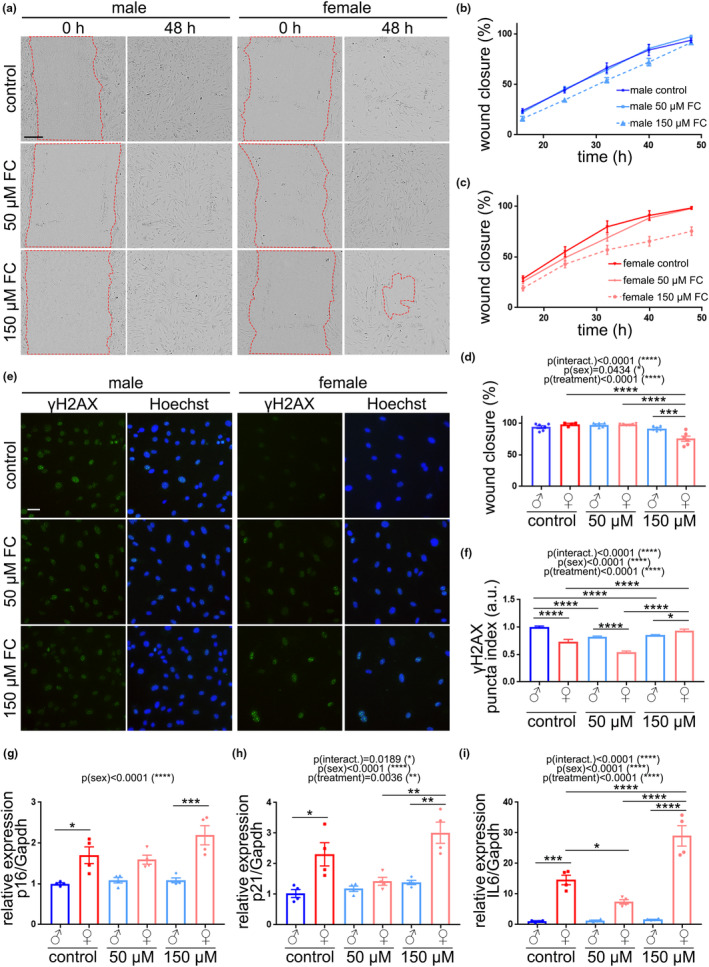
Cultured primary CEC isolated from aged female mice were more susceptible to FC than CEC from male mice. (a) Representative images of the scratch assay in cultured CEC isolated from aged male and female mice and treated with FC (50 or 150 μM), or a vehicle. Images show wound right after (0 h) and 48 h after (48 h) a scratch and treatment initiation. Scale bar, 200 μm. (b,c) Percentage of the wound closure in male CEC (b) and female CEC (c) treated with FC or a vehicle. (d) Percentage of wound closure 48 h after the initiation of the treatments. Data are mean ± SEM pooled from three mice/group. (e) Representative images of cultured CEC from male and female mice treated with FC (50 or 150 μM), or a vehicle. 7 days after the treatments, cells were fixed and stained with anti‐γH2AX (green) and the nuclear Hoechst dye (blue). Scale bar, 25 μm. (f) Puncta index of γH2AX in CEC from (e). Data are mean ± SEM pooled from >500 cells from three mice/group. (g–i) Gene expression of p16 (g), p21 (h), and IL6 (i) relative to Gapdh. Data are mean ± SEM pooled from three mice/group. All experiments were analyzed by two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Then, we wondered if FC may have an effect on cell proliferation, which is inhibited during cell senescence. To study cellular senescence in vitro, cells should be allowed to have at least 7 days after a senescence‐inducing stimulus to become fully senescent (Gonzalez‐Gualda et al., 2021). Thus, in cell proliferation/viability assays, we treated cells with FC (50, or 150 μM), or water as vehicle, for 24 h and 7 days. There were no significant differences 24 h after the treatment (Figure S5). However, 7 days after treatment, control female CEC showed a slight reduction in cell proliferation, compared with corresponding male CEC, and FC treatment significantly reduced cell proliferation in both sexes. Importantly, FC did not induce cell death in CEC derived from both sexes (Figure S6). We also determined if FC can alter autophagy in cultured CEC. For this, we used a construct that codes for an autophagy flux reporter (GFP‐LC3‐RFP) (Kaizuka et al., 2016; Morita et al., 2018). Both at 24 h and 7 d after treatment with FC or vehicle, autophagy flux was significantly reduced in male‐derived cells treated with 150 μM FC, compared with control male cells (Figure S7); however, female‐derived CEC did not exhibit any change in autophagy flux with FC treatment.
FIGURE 6.
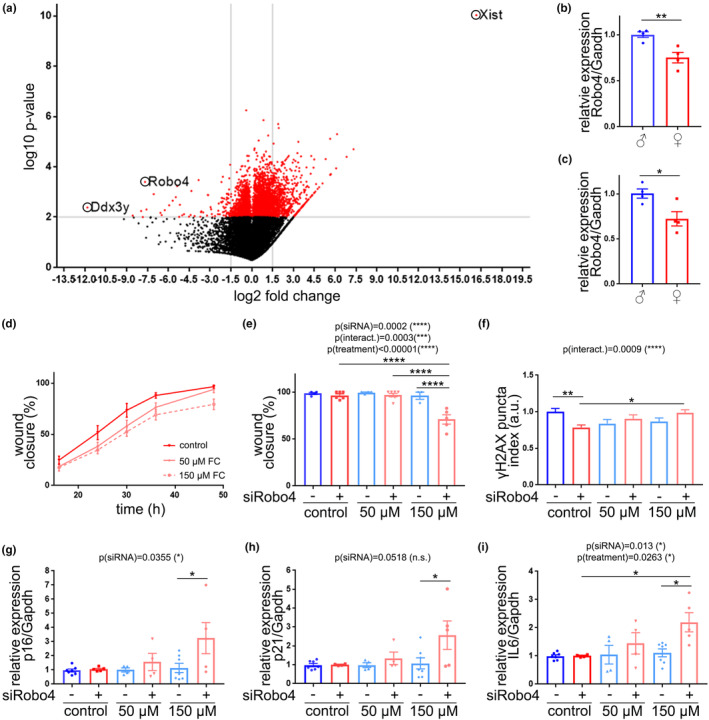
Robo4 downregulation sensitized aged male mice‐derived CEC to FC. (a) Volcano plot showing fold changes for genes differentially expressed between aged female mouse‐derived CEC versus aged male mouse‐derived CEC in culture. (b) Gene expression of Robo4 relative to Gapdh in cultured CEC from aged mice of both sexes. Data are mean ± SEM pooled from three mice/group. Student's t test, *p < 0.05. (c) Gene expression of Robo4 relative to Gapdh in brain microvessel fractions from aged male and female mice. Data are mean ± SEM pooled from three mice/group. Student's t test, *p < 0.05. (d) Cultured CEC from male mice were transfected with siRNA targeting Robo4, or non‐targeting siRNA, and treated with FC, or a vehicle. Line graph shows the percentage of the wound closure every 8 h for 48 h. (e) Percentage of wound closure 48 h after the initiation of the treatments. (f) Puncta index of γH2AX in CEC 7 days after the treatments. Data are mean ± SEM pooled from >250 cells from three mice/group. Two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05, **p < 0.01. (g–i) Gene expression of p16 (g), p21 (h), and IL6 (i) relative to Gapdh. Data are mean ± SEM pooled from two independent experiments from three mice/group. Two‐way ANOVA test, Tukey's multiple comparisons test, *p < 0.05, **p < 0.01.
We determined if FC induces DNA damage in cultured CEC by measuring the puncta index of γH2AX, and observed that sex, treatment and interaction between both factors had significant effect on DNA damage (Figure 5e,f). Female CEC showed reduced γH2AX than male CEC at basal conditions; however, FC induced DNA damage in female CEC, but not in male CEC. Indeed, FC reduced γH2AX in male CEC, suggesting that FC has beneficial effects on male CEC and detrimental in female CEC.
Sex had a significant impact on the relative expression of p16Ink4a (Figure 5g), p21Cip1 (Figure 5h), and IL6 (Figure 5i). The treatment and the interaction had significant effects on p21Cip1 and IL6. Remarkably, 150 μM FC increased the relative expression of p16Ink4a, p21Cip1, and IL6 in CEC derived from female mice compared with male CEC.
Altogether, our data indicate that FC exacerbates the SA phenotype in cultured female mouse‐derived CEC, and that CEC from aged male mice are more resilient to FC treatment.
3.5. Robo4 was downregulated in the cerebrovasculature and in cultured primary CEC derived from aged female mice compared with aged males
We use our RNA‐Seq data to identify candidate genes that could contribute to differences in senescence susceptibility between sexes (Moruno‐Manchon, 2023). In a volcano plot representation (Figure 6a), we observed that the most upregulated gene in aged female CEC is Xist, a non‐coding RNA on the X chromosome that participates in X‐inactivation. The most downregulated gene in aged female CEC is Ddx3y, which codes for an RNA helicase on the Y chromosome. Thus, these two genes confirm the different expression of sex‐linked genes and serve as positive controls for male‐ (Ddx3y) and female‐ (Xist) derived CEC. Robo4 was the second most significantly downregulated gene in female‐derived CEC, compared with male CEC. We confirmed that Robo4 was downregulated in cultured CEC isolated from aged female mice, compared with male CEC (Figure 6b). We also analyzed the relative expression of Robo4 in the microvessel fractions of aged mice, and found that Robo4 was downregulated in aged female mice, compared with aged males (Figure 6c).
3.6. Robo4 downregulation promoted FC‐induced senescence in primary cultured CEC from aged male
ROBO4 (Magic roundabout) is a transmembrane receptor protein that is specifically expressed in endothelial cells and plays a major function in cell proliferation and angiogenesis (Huminiecki et al., 2002). In preclinical studies, Robo4 has also been implicated in preventing senescence induced by TNFα (Tanaka et al., 2017). Thus, we hypothesize that Robo4 downregulation may sensitize endothelial cells to become senescent. Then, we transfected male‐derived CEC with Robo4‐targeting siRNA, or non‐targeting siRNA (Figure S8). Robo4 downregulation significantly reduced the percentage of the wound closure, compared with CEC treated with Robo4‐targeting siRNA, when treated with 150 μM FC (Figure 6d,e). Robo4‐targeting siRNA also negatively affected cell proliferation 24 h and 7 d after the initiation of FC treatment (Figure S8). Furthermore, Robo4 downregulation significantly enhanced DNA damage in CEC treated with 150 μM FC, compared with siRobo4 CEC treated with a vehicle (Figure 6f). Regarding the expression of SA genes, we found that CEC treated with Robo4‐targeting siRNA and 150 μM FC showed significantly upregulated expression of p16Ink4a (Figure 6g), p21Cip1 (Figure 6h), and IL6 (Figure 6i), compared with CEC treated with non‐targeting siRNA and 150 μM FC.
Thus, our data indicate that Robo4 downregulation “sensitizes” male mouse‐derived CEC to enter into a senescent state, as we observed in female mouse‐derived CEC (Figure 5).
4. DISCUSSION
Our study demonstrates that the brains of aged female WT mice are more vulnerable to chronic administration of FC, compared to aged male mice. Aged female mice treated with FC manifest cognitive dysfunction, enhanced iron deposition, impaired autophagy, and enhanced SA‐phenotype in their brain vasculature. In our in vitro experiments, we found that cultured primary CEC isolated from aged female mice and treated with 150 μM FC are more susceptible to show SA phenotype, compared with CEC from aged male mice. Importantly, Robo4 downregulation sensitizes male‐derived CEC to FC treatment to become senescent.
Different models have been used to study the toxicity of iron overload on brain functions. Iron solutions can be injected in the brain by intranigral infusion (Mohanakumar et al., 1994; You et al., 2015), or administered by oral supplementation (Huang et al., 2019; Schroder et al., 2001; Sobotka et al., 1996). Huang et al., used a chronic oral administration of FC (2.5 or 10 mg/day, >4 weeks) in 9 m/o C57BL/6 mice to study the toxicity of iron overload on brain function (Huang et al., 2019). As our bodies incorporate iron only from aliments, we found Huang's model more relevant; however, daily gavage in aged mice resulted in a high mortality independently of the treatment during the first week of treatment, likely because daily gavage is more stressful for aged mice than for young mice. Thus, we performed oral gavage of FC or saline solution alternatively 3 days per week. Huang et al. observed Parkinson‐like phenotype in middle aged male mice treated with FC for 4 and 8 weeks of treatment, compared with control mice. Six weeks after the initiation of the treatment, we observed significant differences in recognition and conditioning memory between FC‐treated female mice and control females, but not between both groups of males. Thus, we found relevant to investigate the molecular mechanisms that may drive to these different outcomes in a sex‐dependent manner, which have not been studied thus far.
Iron deposits have been found in the brain of healthy aged population, and more pronounced in age‐related neurodegenerative disorders (Ndayisaba et al., 2019). Importantly, iron accumulates in specific brain regions and correlates to cognitive dysfunction (Spence et al., 2020). It is still debatable whether iron is a primary cause of dementia or whether iron accumulation in the brain is a secondary effect of brain atrophy. Iron levels in blood are restored at basal levels 6 h after FC administration in rats (Yuan et al., 2017). However, we found that iron levels are enhanced in the brains after chronic administration of FC, suggesting that iron accumulates in the brain tissue during the treatment. Senescence itself can promote iron accumulation (Killilea et al., 2003; Killilea et al., 2004). Thus, aged brains, which show SA phenotype, compared with the brains of young mice (Kiss et al., 2020), may contribute to iron accumulation. In endothelial cells, the transmembrane protein neuropilin‐1 prevents iron accumulation in mitochondria and, thus, mitigates iron‐induced oxidative stress and cell senescence (Issitt et al., 2019). Excess intracellular iron leads to mitochondrial dysfunction (Huang et al., 2009). This suggests that a positive feedback loop exists between iron overload and cell senescence that may aggravate brain homeostasis and contribute to cognitive dysfunction.
We observed a significant increase in the velocity, distance moved, and number of visits to borders, and a significant reduction in the percentage of time not moving in aged female mice, independently of the treatment, compared with aged male mice. Other studies found that aged females show enhanced anxiety‐like behavior than aged male mice (Connolly et al., 2021; McLean et al., 2022). It has been proposed that these sex differences in anxiety‐like behavior and reduced learning may be caused by enhanced expression of pro‐inflammatory molecules (TNFα, IL6) and neuroinflammation in the brain of aged female mice (Connolly et al., 2021; Mangold et al., 2017; Porcher et al., 2021). Overall, aged female mice show more locomotor activity than aged‐matched male mice (Connolly et al., 2021; Garvock‐de Montbrun et al., 2019; Haruyama et al., 2019; McLean et al., 2022), likely due to slightly reduced body weight in female compared with males. However, we did not find significant differences in body weight between sexes. Haruyama et al. proposed that the enhanced spontaneous locomotor activity in aged female mice can be caused by increased expression of an enzyme that prevent guanine oxidation in aged females, but not in males, independently of the body weight (Haruyama et al., 2019). Thus, there exist important sex differences in neuroinflammation and in the transcriptome between sexes that may affect anxiety‐like behavior and locomotor activity in aged mice.
Senescence in the brain vasculature can negatively affect BBB integrity (Yamazaki et al., 2016) and play a significant role in the pathogenesis of vascular dementia (Ueno et al., 2016). The increased levels of circulating markers of endothelial dysfunction are associated with age (50–75 y/o) and with cognitive dysfunction in humans (Heringa et al., 2014). In animal models, 10% of cerebromicrovascular endothelial cells become senescent in 28‐m/o mice (equivalent to 75 y/o in humans). Downregulation of Sirt1, which prevents senescence, in brain endothelial cells has been associated with enhanced permeability of BBB in the brains of aged mice and humans (Stamatovic et al., 2019). Soluble tau aggregates may also be responsible for inducing senescence in the brain vasculature and contribute to Alzheimer disease‐like vasculature deficits in mice (Hussong et al., 2023). This evidence supports that endothelial senescence is a major factor of BBB disruption and vascular dementia.
Our findings highlight sex‐dependent vulnerability to senescence by iron overload in the brain vasculature and in cultured primary CEC derived from aged mice. The major contributing factor to cell senescence is DNA damage (Durik et al., 2012; Yousefzadeh et al., 2021). From the existing literature, female sex is associated with reduced capacity for DNA repair in different organs and cell types (Broestl et al., 2022; Kfoury et al., 2018; Malorni et al., 2008; Rall‐Scharpf et al., 2021; Sun et al., 2014; Trzeciak et al., 2008), with one exception to this assumption found by Yousefzadeh et al. (2020). Walker et al. found that age (>55 y/o) has a greater effect on activating DNA damage response and senescence in aged women compared with aged men (Walker et al., 2016). We found that 150 μM FC significantly increased DNA damage in aged female mouse‐derived CEC, compared with female CEC treated either with 50 μM FC or a vehicle. However, cultured CEC derived from aged male mice showed enhanced DNA damage compared with female mouse‐derived CEC at basal conditions. Martin et al. discussed that we must be cautious with the general assumption that γH2AX represents DNA damage (Martin et al., 2014). Several studies found that γH2AX can occur in absence of DNA damage, and that may occur during replication (Ichijima et al., 2005; MacPhail et al., 2003; McManus & Hendzel, 2005; Tu et al., 2013), suggesting that cells with enhanced ability to proliferate may exhibit enhanced levels of γH2AX, with no correlation with DNA damage. Thus, measuring only DNA damage markers does not guarantee a positive correlation with senescence. From our data and considering also the reduced the expression of p16, p21, and IL6, male‐derived CEC are more resilient to FC‐induced senescence than female CEC.
Autophagy is an intracellular process that degrades and recycles cellular components as an adaptive strategy to environmental changes (Klionsky et al., 2021). Autophagy maintains the integrity of the BBB by regulating the levels of tight junction proteins, such as claudin‐5 (Yang et al., 2019). Either reduction (Hyun & Jung, 2014; Kaur et al., 2011; Rom et al., 2020) or accumulation (Feng et al., 2018; Gholami et al., 2022; Kakogiannos et al., 2020) of claudin‐5 results in the impairment of the BBB. Claudin‐5 localizes in the endothelial cell membrane to maintain the BBB permeability in conjunction with ZO‐1 (Jiao et al., 2011). However, it can be internalized in the cytosol by caveolin‐1‐mediated endocytosis; thus, being claudin‐5 excluded from brain endothelial borders, which causes BBB disruption (Liu et al., 2012). In the cytosol, claudin‐5 is degraded by autophagy. Indeed, autophagy eliminates aggregates of claudin‐5 that accumulate in the cytosol during hypoxia (Liu et al., 2016; Yang et al., 2021; Yu et al., 2021) or infections (Lin et al., 2022). However, autophagy impairment leads to accumulation of claudin‐5 in the cytosol of endothelial cells and impairs BBB (Liu et al., 2016; Yang et al., 2019). Importantly, iron overload negatively affects autophagy (Jahng et al., 2019; Uberti et al., 2020). We observed that autophagy was impaired by chronic administration of FC in aged female mice. Similarly, claudin‐5 levels were enhanced in the brains of aged female mice treated with FC, but not in male mice. Thus, autophagy impairment by FC may lead to claudin‐5 accumulation, and this could lead to BBB breakdown.
ROBO4 is a transmembrane receptor protein that is specifically expressed in endothelial cells (Huminiecki et al., 2002). ROBO4 is mostly known as a regulator of endothelial cell migration and proliferation (Dai et al., 2019). Dysregulated expression of ROBO4, either upregulation or downregulation, has been importantly associated with angiogenesis sites and in cancer tissues (Huminiecki et al., 2002; Yamanaka et al., 2022; Yeo et al., 2022). In preclinical studies, Robo4 downregulation enhances permeability in cultured endothelial cells (Bekes et al., 2017; Cai et al., 2015). Robo4 has also been involved in pro‐inflammatory responses that ultimately lead to cell senescence. The pro‐inflammatory cytokine TNF‐α can promote the activation of NF‐κB, which binds to the promoter of Robo4, and thus enhances the expression of Robo4, likely as a compensatory mechanism to mitigate deleterious effects of TNFα (Tanaka et al., 2017). Robo4 interacts with TNFreceptor‐associated factor 7 and this complex can prevent hyperpermeability induced by TNF‐α by inhibiting cytoplasmic internalization of the adherens junction protein VE‐cadherin (Shirakura et al., 2019). We found that IL6 is upregulated in female mouse‐derived CEC and in Robo4 downregulated male‐derived CEC treated with 150 μM FC, which supports the anti‐inflammatory role of Robo4. However, a study found that Robo4 downregulation prevents LPS‐induced IL6 production by inhibiting the synthesis of granulocyte macrophage colony‐stimulating factor (Shirakura et al., 2018). Thus, Robo4 may have a dual role in inflammatory responses likely depending on cellular environments.
Our study provides evidence that sex differences exist in the brain function and, particularly, in the brain vasculature of aged mice with chronic administration of FC, and that Robo4 downregulation is a senescence‐inducing factor in combination with FC. This study highlights the necessity to evaluate the risk of brain vascular impairment and dementia in female patients and in patients with ROBO4 variants. Importantly, developing therapeutical approaches to prevent endothelial senescence is a strategy to protect brain vasculature and mitigate vascular dementia in the elderly population.
AUTHOR CONTRIBUTIONS
Brian Noh isolated and cultured CEC and performed the majority of experiments and gathered data. Maria Pilar Blasco‐Conesa, Syed Mushfiqur Rahman, and Sheelu Monga performed individual experiments and analyzed data. Gary Guzman and Rodney Ritzel performed flow cytometry experiments. Yun‐Ju Lai processed CEC samples for RNA‐seq. Bhanu Priya Ganesh, Akihiko Urayama, and Louise D. McCullough advised on the study. Jose Felix Moruno‐Manchon designed the study, prepared figures, and wrote the manuscript.
FUNDING INFORMATION
This research was conducted with the financial support to the J.F.M.M.'s lab from the NIA (R21AG075750, J.F.M.M.), the Texas Alzheimer's Research and Care Consortium (#957578, J.F.M.M.), the American Heart Association (#856061, J.F.M.M.), and start‐up funds from the University of Texas Health Science Center at Houston McGovern Medical School (J.F.M.M).
CONFLICT OF INTEREST STATEMENT
All authors declare no competing financial interests.
Supporting information
FigureS1
FigureS2
FigureS3
FigureS4
FigureS5
FigureS6
FigureS7
FigureS8
TableS1
ACKNOWLEDGMENTS
We thank Lori Capozzi and Michael Maniskas for administrative support.
Noh, B. , Blasco‐Conesa, M. P. , Rahman, S. M. , Monga, S. , Ritzel, R. , Guzman, G. , Lai, Y.‐J. , Ganesh, B. P. , Urayama, A. , McCullough, L. D. , & Moruno‐Manchon, J. F. (2023). Iron overload induces cerebral endothelial senescence in aged mice and in primary culture in a sex‐dependent manner. Aging Cell, 22, e13977. 10.1111/acel.13977
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Harvard Dataverse at https://doi.org/10.7910/DVN/B9YKGC.
REFERENCES
- Algarin, C. , Karunakaran, K. D. , Reyes, S. , Morales, C. , Lozoff, B. , Peirano, P. , & Biswal, B. (2017). Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Frontiers in Aging Neuroscience, 9, 54. 10.3389/fnagi.2017.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova, D. M. , & Brown, D. R. (2018). Altered processing of beta‐amyloid in SH‐SY5Y cells induced by model senescent microglia. ACS Chemical Neuroscience, 9(12), 3137–3152. 10.1021/acschemneuro.8b00334 [DOI] [PubMed] [Google Scholar]
- Bekes, I. , Haunerdinger, V. , Sauter, R. , Holzheu, I. , Janni, W. , Wöckel, A. , & Wulff, C. (2017). Slit2/Robo4 signaling: Potential role of a VEGF‐antagonist pathway to regulate luteal permeability. Geburtshilfe Und Frauenheilkunde, 77(1), 73–80. 10.1055/s-0042-113461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, G. A. , Fishbane, S. , Rodriguez, M. , Smits, G. , Shemesh, S. , Pergola, P. E. , Wolf, M. , & Chertow, G. M. (2015). A 12‐week, double‐blind, placebo‐controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3‐5. American Journal of Kidney Diseases, 65(5), 728–736. 10.1053/j.ajkd.2014.10.014 [DOI] [PubMed] [Google Scholar]
- Broadbent, N. J. , Squire, L. R. , & Clark, R. E. (2004). Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 101(40), 14515–14520. 10.1073/pnas.0406344101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broestl, L. , Warrington, N. M. , Grandison, L. , Abou‐Antoun, T. , Tung, O. , Shenoy, S. , Tallman, M. M. , Rhee, G. , Yang, W. , Sponagel, J. , Yang, L. , Kfoury‐Beaumont, N. , Hill, C. M. , Qanni, S. A. , Mao, D. D. , Kim, A. H. , Stewart, S. A. , Venere, M. , Luo, J. , & Rubin, J. B. (2022). Gonadal sex patterns p21‐induced cellular senescence in mouse and human glioblastoma. Commun Biol, 5(1), 781. 10.1038/s42003-022-03743-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, H. , Liu, W. , Xue, Y. , Shang, X. , Liu, J. , Li, Z. , Wang, P. , Liu, L. , Hu, Y. , & Liu, Y. (2015). Roundabout 4 regulates blood‐tumor barrier permeability through the modulation of ZO‐1, Occludin, and Claudin‐5 expression. Journal of Neuropathology and Experimental Neurology, 74(1), 25–37. 10.1097/NEN.0000000000000146 [DOI] [PubMed] [Google Scholar]
- Cayo, A. , Segovia, R. , Venturini, W. , Moore‐Carrasco, R. , Valenzuela, C. , & Brown, N. (2021). mTOR activity and autophagy in senescent cells, a complex partnership. International Journal of Molecular Sciences, 22(15), 8149. 10.3390/ijms22158149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly, M. G. , Potter, O. V. , Sexton, A. R. , & Kohman, R. A. (2021). Effects of toll‐like receptor 4 inhibition on spatial memory and cell proliferation in male and female adult and aged mice. Brain, Behavior, and Immunity, 97, 383–393. 10.1016/j.bbi.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi, A. , Orellana, D. I. , Santambrogio, P. , Rubio, A. , Cancellieri, C. , Giannelli, S. , Ripamonti, M. , Taverna, S. , di Lullo, G. , Rovida, E. , Ferrari, M. , Forni, G. L. , Fiorillo, C. , Broccoli, V. , & Levi, S. (2019). Stem cell modeling of Neuroferritinopathy reveals iron as a determinant of senescence and Ferroptosis during neuronal aging. Stem Cell Reports, 13(5), 832–846. 10.1016/j.stemcr.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, A. R. , Fey, C. , Morris, C. M. , Bindoff, L. A. , Ince, P. G. , Chinnery, P. F. , Coulthard, A. , Jackson, M. J. , Jackson, A. P. , McHale, D. P. , Hay, D. , Barker, W. A. , Markham, A. F. , Bates, D. , Curtis, A. , & Burn, J. (2001). Mutation in the gene encoding ferritin light polypeptide causes dominant adult‐onset basal ganglia disease. Nature Genetics, 28(4), 350–354. 10.1038/ng571 [DOI] [PubMed] [Google Scholar]
- Dai, C. , Gong, Q. , Cheng, Y. , & Su, G. (2019). Regulatory mechanisms of Robo4 and their effects on angiogenesis. Bioscience Reports, 39(7), BSR20190513. 10.1042/BSR20190513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del C Valdés Hernández, M. , Ritchie, S. , Glatz, A. , Allerhand, M. , Muñoz Maniega, S. , Gow, A. J. , Royle, N. A. , Bastin, M. E. , Starr, J. M. , Deary, I. J. , & Wardlaw, J. M. (2015). Brain iron deposits and lifespan cognitive ability. Age (Dordrecht, Netherlands), 37(5), 100. 10.1007/s11357-015-9837-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle, J. , Duran‐Vilaregut, J. , Manich, G. , Camins, A. , Pallàs, M. , Vilaplana, J. , & Pelegrí, C. (2009). Time‐course of blood‐brain barrier disruption in senescence‐accelerated mouse prone 8 (SAMP8) mice. International Journal of Developmental Neuroscience, 27(1), 47–52. 10.1016/j.ijdevneu.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Ding, W. , Gao, S. , & Scott, R. E. (2001). Senescence represses the nuclear localization of the serum response factor and differentiation regulates its nuclear localization with lineage specificity. Journal of Cell Science, 114(Pt 5), 1011–1018. 10.1242/jcs.114.5.1011 [DOI] [PubMed] [Google Scholar]
- Durik, M. , Kavousi, M. , van der Pluijm, I. , Isaacs, A. , Cheng, C. , Verdonk, K. , Loot, A. E. , Oeseburg, H. , Bhaggoe, U. M. , Leijten, F. , Van Veghel, R. , De Vries, R. , Rudez, G. , Brandt, R. , Ridwan, Y. R. , Van Deel, E. D. , De Boer, M. , Tempel, D. , Fleming, I. , … Roks, A. J. M. (2012). Nucleotide excision DNA repair is associated with age‐related vascular dysfunction. Circulation, 126(4), 468–478. 10.1161/CIRCULATIONAHA.112.104380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, S. , Zou, L. , Wang, H. , He, R. , Liu, K. , & Zhu, H. (2018). RhoA/ROCK‐2 pathway inhibition and tight junction protein upregulation by Catalpol suppresses Lipopolysaccaride‐induced disruption of blood‐brain barrier permeability. Molecules, 23(9), 2371. 10.3390/molecules23092371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbane, S. , Block, G. A. , Loram, L. , Neylan, J. , Pergola, P. E. , Uhlig, K. , & Chertow, G. M. (2017). Effects of ferric citrate in patients with nondialysis‐dependent CKD and iron deficiency anemia. J Am Soc Nephrol, 28(6), 1851–1858. 10.1681/ASN.2016101053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahche, J. J. , Bailey, R. L. , Potischman, N. , & Dwyer, J. T. (2017). Dietary supplement use was very high among older adults in the United States in 2011‐2014. The Journal of Nutrition, 147(10), 1968–1976. 10.3945/jn.117.255984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvock‐de Montbrun, T. , Fertan, E. , Stover, K. , & Brown, R. E. (2019). Motor deficits in 16‐month‐old male and female 3xTg‐AD mice. Behavioural Brain Research, 356, 305–313. 10.1016/j.bbr.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Gholami, L. , Jokar, S. , Fatahi, Y. , Samandari, H. , Hamzehalipour Almaki, J. , Hosseini, M. , & Parviz, M. (2022). Targeting Caveolin‐1 and Claudin‐5 with AY9944, improve blood‐brain barrier permeability; computational simulation and experimental study. Cellular and Molecular Neurobiology, 42(4), 1125–1139. 10.1007/s10571-020-01004-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Gualda, E. , Baker, A. G. , Fruk, L. , & Munoz‐Espin, D. (2021). A guide to assessing cellular senescence in vitro and in vivo. The FEBS Journal, 288(1), 56–80. 10.1111/febs.15570 [DOI] [PubMed] [Google Scholar]
- Graves, S. I. , & Baker, D. J. (2020). Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic & Clinical Pharmacology & Toxicology, 127(2), 102–110. 10.1111/bcpt.13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A. (2014). Ferric citrate hydrate as a phosphate binder and risk of aluminum toxicity. Pharmaceuticals (Basel), 7(10), 990–998. 10.3390/ph7100990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawi, R. , Moukhadder, H. , & Taher, A. (2017). Anemia in the elderly: A consequence of aging? Expert Review of Hematology, 10(4), 327–335. 10.1080/17474086.2017.1285695 [DOI] [PubMed] [Google Scholar]
- Hare, D. , Ayton, S. , Bush, A. , & Lei, P. (2013). A delicate balance: Iron metabolism and diseases of the brain. Frontiers in Aging Neuroscience, 5, 34. 10.3389/fnagi.2013.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama, N. , Sakumi, K. , Katogi, A. , Tsuchimoto, D. , De Luca, G. , Bignami, M. , & Nakabeppu, Y. (2019). 8‐Oxoguanine accumulation in aged female brain impairs neurogenesis in the dentate gyrus and major Island of Calleja, causing sexually dimorphic phenotypes. Progress in Neurobiology, 180, 101613. 10.1016/j.pneurobio.2019.04.002 [DOI] [PubMed] [Google Scholar]
- He, Z. Y. , Wang, W. Y. , Hu, W. Y. , Yang, L. , Li, Y. , Zhang, W. Y. , Yang, Y. S. , Liu, S. C. , Zhang, F. L. , Mei, R. , Xing, D. , Xiao, Z. C. , & Zhang, M. (2016). Gamma‐H2AX upregulation caused by Wip1 deficiency increases depression‐related cellular senescence in hippocampus. Scientific Reports, 6, 34558. 10.1038/srep34558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringa, S. M. , van den Berg, E. , Reijmer, Y. D. , Nijpels, G. , Stehouwer, C. D. , Schalkwijk, C. G. , Teerlink, T. , Scheffer, P. G. , van den Hurk, K. , Kappelle, L. J. , Dekker, J. M. , & Biessels, G. J. (2014). Markers of low‐grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population ‐ the Hoorn study. Psychoneuroendocrinology, 40, 108–118. 10.1016/j.psyneuen.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Hu, H. , Ji, Q. , Song, M. , Ren, J. , Liu, Z. , Wang, Z. , Liu, X. , Yan, K. , Hu, J. , Jing, Y. , Wang, S. , Zhang, W. , Liu, G. H. , & Qu, J. (2020). ZKSCAN3 counteracts cellular senescence by stabilizing heterochromatin. Nucleic Acids Research, 48(11), 6001–6018. 10.1093/nar/gkaa425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Ma, W. , Luo, Q. , Shi, L. , Xia, Y. , Lao, C. , Liu, W. , Zou, Y. , Cheng, A. , Shi, R. , & Chen, Z. (2019). Iron overload resulting from the chronic oral administration of ferric citrate induces parkinsonism phenotypes in middle‐aged mice. Aging (Albany NY), 11(21), 9846–9861. 10.18632/aging.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. L. , Becker, E. M. , Whitnall, M. , Suryo Rahmanto, Y. , Ponka, P. , & Richardson, D. R. (2009). Elucidation of the mechanism of mitochondrial iron loading in Friedreich's ataxia by analysis of a mouse mutant. Proceedings of the National Academy of Sciences of the United States of America, 106(38), 16381–16386. 10.1073/pnas.0906784106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huminiecki, L. , Gorn, M. , Suchting, S. , Poulsom, R. , & Bicknell, R. (2002). Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics, 79(4), 547–552. 10.1006/geno.2002.6745 [DOI] [PubMed] [Google Scholar]
- Hussong, S. A. , Banh, A. Q. , van Skike, C. , Dorigatti, A. O. , Hernandez, S. F. , Hart, M. J. , Ferran, B. , Makhlouf, H. , Gaczynska, M. , Osmulski, P. A. , McAllen, S. , Dineley, K. T. , Ungvari, Z. , Perez, V. I. , Kayed, R. , & Galvan, V. (2023). Soluble pathogenic tau enters brain vascular endothelial cells and drives cellular senescence and brain microvascular dysfunction in a mouse model of tauopathy. Nature Communications, 14(1), 2367. 10.1038/s41467-023-37840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, S. W. , & Jung, Y. S. (2014). Hypoxia induces FoxO3a‐mediated dysfunction of blood‐brain barrier. Biochemical and Biophysical Research Communications, 450(4), 1638–1642. 10.1016/j.bbrc.2014.07.055 [DOI] [PubMed] [Google Scholar]
- Ichijima, Y. , Sakasai, R. , Okita, N. , Asahina, K. , Mizutani, S. , & Teraoka, H. (2005). Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochemical and Biophysical Research Communications, 336(3), 807–812. 10.1016/j.bbrc.2005.08.164 [DOI] [PubMed] [Google Scholar]
- Issitt, T. , Bosseboeuf, E. , de Winter, N. , Dufton, N. , Gestri, G. , Senatore, V. , Chikh, A. , Randi, A. M. , & Raimondi, C. (2019). Neuropilin‐1 controls endothelial homeostasis by regulating mitochondrial function and iron‐dependent oxidative stress. iScience, 11, 205–223. 10.1016/j.isci.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng, J. W. S. , Alsaadi, R. M. , Palanivel, R. , Song, E. , Hipolito, V. E. B. , Sung, H. K. , Botelho, R. J. , Russell, R. C. , & Sweeney, G. (2019). Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Reports, 20(10), e47911. 10.15252/embr.201947911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, H. , Wang, Z. , Liu, Y. , Wang, P. , & Xue, Y. (2011). Specific role of tight junction proteins claudin‐5, occludin, and ZO‐1 of the blood‐brain barrier in a focal cerebral ischemic insult. Journal of Molecular Neuroscience, 44(2), 130–139. 10.1007/s12031-011-9496-4 [DOI] [PubMed] [Google Scholar]
- Kaizuka, T. , Morishita, H. , Hama, Y. , Tsukamoto, S. , Matsui, T. , Toyota, Y. , Kodama, A. , Ishihara, T. , Mizushima, T. , & Mizushima, N. (2016). An Autophagic flux probe that releases an internal control. Molecular Cell, 64(4), 835–849. 10.1016/j.molcel.2016.09.037 [DOI] [PubMed] [Google Scholar]
- Kakogiannos, N. , Ferrari, L. , Giampietro, C. , Scalise, A. A. , Maderna, C. , Ravà, M. , Taddei, A. , Lampugnani, M. G. , Pisati, F. , Malinverno, M. , Martini, E. , Costa, I. , Lupia, M. , Cavallaro, U. , Beznoussenko, G. V. , Mironov, A. A. , Fernandes, B. , Rudini, N. , Dejana, E. , & Giannotta, M. (2020). JAM‐A acts via C/EBP‐alpha to promote Claudin‐5 expression and enhance endothelial barrier function. Circulation Research, 127(8), 1056–1073. 10.1161/CIRCRESAHA.120.316742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, J. , Tuor, U. I. , Zhao, Z. , & Barber, P. A. (2011). Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood‐brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. Journal of Cerebral Blood Flow and Metabolism, 31(9), 1874–1885. 10.1038/jcbfm.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfoury, N. , Sun, T. , Yu, K. , Rockwell, N. , Tinkum, K. L. , Qi, Z. , Warrington, N. M. , McDonald, P. , Roy, A. , Weir, S. J. , Mohila, C. A. , Deneen, B. , & Rubin, J. B. (2018). Cooperative p16 and p21 action protects female astrocytes from transformation. Acta Neuropathologica Communications, 6(1), 12. 10.1186/s40478-018-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea, D. W. , Atamna, H. , Liao, C. , & Ames, B. N. (2003). Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxidants & Redox Signaling, 5(5), 507–516. 10.1089/152308603770310158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea, D. W. , Wong, S. L. , Cahaya, H. S. , Atamna, H. , & Ames, B. N. (2004). Iron accumulation during cellular senescence. Annals of the new York Academy of Sciences, 1019, 365–367. 10.1196/annals.1297.063 [DOI] [PubMed] [Google Scholar]
- Kim, W. B. , & Cho, J. H. (2020). Encoding of contextual fear memory in hippocampal‐amygdala circuit. Nature Communications, 11(1), 1382. 10.1038/s41467-020-15121-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, T. , Nyúl‐Tóth, Á. , Balasubramanian, P. , Tarantini, S. , Ahire, C. , DelFavero, J. , Yabluchanskiy, A. , Csipo, T. , Farkas, E. , Wiley, G. , Garman, L. , Csiszar, A. , & Ungvari, Z. (2020). Single‐cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience, 42(2), 429–444. 10.1007/s11357-020-00177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, D. J. , Abdel‐Aziz, A. K. , Abdelfatah, S. , Abdellatif, M. , Abdoli, A. , Abel, S. , Abeliovich, H. , Abildgaard, M. H. , Abudu, Y. P. , Acevedo‐Arozena, A. , Adamopoulos, I. E. , Adeli, K. , Adolph, T. E. , Adornetto, A. , Aflaki, E. , Agam, G. , Agarwal, A. , Aggarwal, B. B. , Agnello, M. , … Tong, C. K. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy, 17(1), 1–382. 10.1080/15548627.2020.1797280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. P. , Emechebe, U. , Smith, R. , Franklin, S. , Moore, B. , Yandell, M. , Lessnick, S. L. , & Moon, A. M. (2014). Coordinated control of senescence by lncRNA and a novel T‐box3 co‐repressor complex. eLife, 3, e02805. 10.7554/eLife.02805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. T. , Lee, C. C. , Wu, M. J. , Chiu, Y. W. , Leu, J. G. , Wu, M. S. , Peng, Y. S. , Wu, M. S. , & Tarng, D. C. (2022). Long‐term safety and efficacy of ferric citrate in phosphate‐lowering and iron‐repletion effects among patients with on hemodialysis: A multicenter, open‐label, phase IV trial. PLoS One, 17(3), e0264727. 10.1371/journal.pone.0264727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. E. , & Bar‐Sagi, D. (2010). Oncogenic KRas suppresses inflammation‐associated senescence of pancreatic ductal cells. Cancer Cell, 18(5), 448–458. 10.1016/j.ccr.2010.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Ji, C. , Xuan, W. , Chen, W. , Lv, Y. , Liu, T. , You, Y. , Gao, F. , Zheng, Q. , & Shao, J. (2021). Effects of daily iron supplementation on motor development and brain connectivity in preterm infants: A diffusion magnetic resonance study. Frontiers in Neuroscience, 15, 769558. 10.3389/fnins.2021.769558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Kim, J. , Buckett, P. D. , Bohlke, M. , Maher, T. J. , & Wessling‐Resnick, M. (2011). Severe postnatal iron deficiency alters emotional behavior and dopamine levels in the prefrontal cortex of young male rats. The Journal of Nutrition, 141(12), 2133–2138. 10.3945/jn.111.145946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, P. , Tan, R. , Yu, P. , Li, Y. , Mo, Y. , Li, W. , & Zhang, J. (2022). Autophagic degradation of claudin‐5 mediated by its binding to a Clostridium perfringens enterotoxin fragment modulates endothelial barrier permeability. FEBS Letters, 596(7), 924–937. 10.1002/1873-3468.14315 [DOI] [PubMed] [Google Scholar]
- Lin, Y. F. , Wang, L. Y. , Chen, C. S. , Li, C. C. , & Hsiao, Y. H. (2021). Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress. Neurobiol Stress, 15, 100341. 10.1016/j.ynstr.2021.100341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Jin, X. , Liu, K. J. , & Liu, W. (2012). Matrix metalloproteinase‐2‐mediated occludin degradation and caveolin‐1‐mediated claudin‐5 redistribution contribute to blood‐brain barrier damage in early ischemic stroke stage. The Journal of Neuroscience, 32(9), 3044–3057. 10.1523/JNEUROSCI.6409-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Weaver, J. , Jin, X. , Zhang, Y. , Xu, J. , Liu, K. J. , Li, W. , & Liu, W. (2016). Nitric oxide interacts with Caveolin‐1 to facilitate autophagy‐lysosome‐mediated Claudin‐5 degradation in oxygen‐glucose deprivation‐treated endothelial cells. Molecular Neurobiology, 53(9), 5935–5947. 10.1007/s12035-015-9504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Matsui, T. S. , Kang, N. , & Deguchi, S. (2022). Analysis of senescence‐responsive stress fiber proteome reveals reorganization of stress fibers mediated by elongation factor eEF2 in HFF‐1 cells. Molecular Biology of the Cell, 33(1), ar10. 10.1091/mbc.E21-05-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Pi, C. , Yang, Y. , Lin, L. , Shi, Y. , Li, Y. , Li, Y. , & He, X. (2017). Nampt expression decreases age‐related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS One, 12(1), e0170930. 10.1371/journal.pone.0170930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail, S. H. , Banath, J. P. , Yu, Y. , Chu, E. , & Olive, P. L. (2003). Cell cycle‐dependent expression of phosphorylated histone H2AX: Reduced expression in unirradiated but not X‐irradiated G1‐phase cells. Radiation Research, 159(6), 759–767. 10.1667/rr3003 [DOI] [PubMed] [Google Scholar]
- Malorni, W. , Straface, E. , Matarrese, P. , Ascione, B. , Coinu, R. , Canu, S. , Galluzzo, P. , Marino, M. , & Franconi, F. (2008). Redox state and gender differences in vascular smooth muscle cells. FEBS Letters, 582(5), 635–642. 10.1016/j.febslet.2008.01.034 [DOI] [PubMed] [Google Scholar]
- Mangold, C. A. , Wronowski, B. , du, M. , Masser, D. R. , Hadad, N. , Bixler, G. V. , Brucklacher, R. M. , Ford, M. M. , Sonntag, W. E. , & Freeman, W. M. (2017). Sexually divergent induction of microglial‐associated neuroinflammation with hippocampal aging. Journal of Neuroinflammation, 14(1), 141. 10.1186/s12974-017-0920-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, F. , Sousa, J. C. , Sousa, N. , & Palha, J. A. (2013). Blood‐brain‐barriers in aging and in Alzheimer's disease. Molecular Neurodegeneration, 8, 38. 10.1186/1750-1326-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, M. , Terradas, M. , Hernandez, L. , & Genesca, A. (2014). gammaH2AX foci on apparently intact mitotic chromosomes: Not signatures of misrejoining events but signals of unresolved DNA damage. Cell Cycle, 13(19), 3026–3036. 10.4161/15384101.2014.947786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaldan, S. , Clatworthy, S. A. S. , Gamell, C. , Meggyesy, P. M. , Rigopoulos, A. T. , Haupt, S. , Haupt, Y. , Denoyer, D. , Adlard, P. A. , Bush, A. I. , & Cater, M. A. (2018). Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biology, 14, 100–115. 10.1016/j.redox.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, J. W. , Bhattrai, A. , Vitali, F. , Raikes, A. C. , Wiegand, J. L. , & Brinton, R. D. (2022). Contributions of sex and genotype to exploratory behavior differences in an aged humanized APOE mouse model of late‐onset Alzheimer's disease. Learning & Memory, 29(9), 321–331. 10.1101/lm.053588.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, K. J. , & Hendzel, M. J. (2005). ATM‐dependent DNA damage‐independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Molecular Biology of the Cell, 16(10), 5013–5025. 10.1091/mbc.e05-01-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanakumar, K. P. , de Bartolomeis, A. , Wu, R. M. , Yeh, K. J. , Sternberger, L. M. , Peng, S. Y. , Murphy, D. L. , & Chiueh, C. C. (1994). Ferrous‐citrate complex and nigral degeneration: Evidence for free‐radical formation and lipid peroxidation. Annals of the new York Academy of Sciences, 738, 392–399. 10.1111/j.1749-6632.1994.tb21828.x [DOI] [PubMed] [Google Scholar]
- Morita, K. , Hama, Y. , Izume, T. , Tamura, N. , Ueno, T. , Yamashita, Y. , Sakamaki, Y. , Mimura, K. , Morishita, H. , Shihoya, W. , Nureki, O. , Mano, H. , & Mizushima, N. (2018). Genome‐wide CRISPR screen identifies TMEM41B as a gene required for autophagosome formation. The Journal of Cell Biology, 217(11), 3817–3828. 10.1083/jcb.201804132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruno‐Manchon, J. F. (2023). RNAseq primary CEC derived from aged male and female mice. 10.7910/DVN/B9YKGC [DOI]
- Moruno‐Manchon, J. F. , Uzor, N. E. , Blasco‐Conesa, M. P. , Mannuru, S. , Putluri, N. , Furr‐Stimming, E. E. , & Tsvetkov, A. S. (2017). Inhibiting sphingosine kinase 2 mitigates mutant huntingtin‐induced neurodegeneration in neuron models of Huntington disease. Human Molecular Genetics, 26(7), 1305–1317. 10.1093/hmg/ddx046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndayisaba, A. , Kaindlstorfer, C. , & Wenning, G. K. (2019). Iron in neurodegeneration ‐ cause or consequence? Frontiers in Neuroscience, 13, 180. 10.3389/fnins.2019.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi, M. , Saraya, A. , Mochizuki, S. , Helin, K. , Koseki, H. , & Iwama, A. (2010). A novel zinc finger protein Zfp277 mediates transcriptional repression of the Ink4a/arf locus through polycomb repressive complex 1. PLoS One, 5(8), e12373. 10.1371/journal.pone.0012373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen, E. , de Vry, J. , Antonides, A. , Paes, D. , Schepers, M. , van der Staay, F. J. , Prickaerts, J. , & Vanmierlo, T. (2017). Early‐postnatal iron deficiency impacts plasticity in the dorsal and ventral hippocampus in piglets. International Journal of Developmental Neuroscience, 59, 47–51. 10.1016/j.ijdevneu.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Noh, B. , Blasco‐Conesa, M. P. , Lai, Y.‐J. , Ganesh, B. P. , Urayama, A. , Moreno‐Gonzalez, I. , Marrelli, S. P. , McCullough, L. D. , & Moruno‐Manchon, J. F. (2022). G‐quadruplexes stabilization upregulates CCN1 and accelerates aging in cultured cerebral endothelial cells. Frontiers in Aging, 2, 797562. 10.3389/fragi.2021.797562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, S. B. V. , Barroso, S. C. C. , Bicalho, M. A. C. , & Reis, A. M. M. (2018). Profile of drugs used for self‐medication by elderly attended at a referral center. Einstein (Sao Paulo), 16(4), eAO4372. 10.31744/einstein_journal/2018AO4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrí, C. , Canudas, A. M. , del Valle, J. , Casadesus, G. , Smith, M. A. , Camins, A. , Pallàs, M. , & Vilaplana, J. (2007). Increased permeability of blood‐brain barrier on the hippocampus of a murine model of senescence. Mechanisms of Ageing and Development, 128(9), 522–528. 10.1016/j.mad.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Porcher, L. , Bruckmeier, S. , Burbano, S. D. , Finnell, J. E. , Gorny, N. , Klett, J. , Wood, S. K. , & Kelly, M. P. (2021). Aging triggers an upregulation of a multitude of cytokines in the male and especially the female rodent hippocampus but more discrete changes in other brain regions. Journal of Neuroinflammation, 18(1), 219. 10.1186/s12974-021-02252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran, P. , Alzahrani, A. M. , Hanieh, H. N. , Kumar, S. A. , Ben Ammar, R. , Rengarajan, T. , & Alhoot, M. A. (2019). Autophagy and senescence: A new insight in selected human diseases. Journal of Cellular Physiology, 234(12), 21485–21492. 10.1002/jcp.28895 [DOI] [PubMed] [Google Scholar]
- Rall‐Scharpf, M. , Friedl, T. W. P. , Biechonski, S. , Denkinger, M. , Milyavsky, M. , & Wiesmuller, L. (2021). Sex‐specific differences in DNA double‐strand break repair of cycling human lymphocytes during aging. Aging (Albany NY), 13(17), 21066–21089. 10.18632/aging.203519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel, R. M. , Li, Y. , Lei, Z. , Carter, J. , He, J. , Choi, H. M. C. , Khan, N. , Li, H. , Allen, S. , Lipinski, M. M. , Faden, A. I. , & Wu, J. (2022). Functional and transcriptional profiling of microglial activation during the chronic phase of TBI identifies an age‐related driver of poor outcome in old mice. Geroscience, 44(3), 1407–1440. 10.1007/s11357-022-00562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom, S. , Heldt, N. A. , Gajghate, S. , Seliga, A. , Reichenbach, N. L. , & Persidsky, Y. (2020). Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin‐5 protein secretion on extracellular microvesicles. Scientific Reports, 10(1), 7274. 10.1038/s41598-020-64349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas, M. , Gregory, F. , Jones, R. , Poolman, R. , Starborg, M. , Rowe, J. , Brookes, S. , & Peters, G. (2007). CDK4 and CDK6 delay senescence by kinase‐dependent and p16INK4a‐independent mechanisms. Molecular and Cellular Biology, 27(12), 4273–4282. 10.1128/MCB.02286-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T. , Shapiro, J. S. , Chang, H. C. , Miller, R. A. , & Ardehali, H. (2022). Aging is associated with increased brain iron through cortex‐derived hepcidin expression. eLife, 11, e73456. 10.7554/eLife.73456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder, N. , Fredriksson, A. , Vianna, M. R. , Roesler, R. , Izquierdo, I. , & Archer, T. (2001). Memory deficits in adult rats following postnatal iron administration. Behavioural Brain Research, 124(1), 77–85. 10.1016/s0166-4328(01)00236-4 [DOI] [PubMed] [Google Scholar]
- Shirakura, K. , Ishiba, R. , Kashio, T. , Funatsu, R. , Tanaka, T. , Fukada, S. I. , Ishimoto, K. , Hino, N. , Kondoh, M. , Ago, Y. , Fujio, Y. , Yano, K. , Doi, T. , Aird, W. C. , & Okada, Y. (2019). The Robo4‐TRAF7 complex suppresses endothelial hyperpermeability in inflammation. Journal of Cell Science, 132(1), jcs220228. 10.1242/jcs.220228 [DOI] [PubMed] [Google Scholar]
- Shirakura, K. , Ishiba, R. , Kashio, T. , Sakai, M. , Fukushima, Y. , Yamamoto, N. , Manabe, S. , Shigesada, N. , Tanaka, T. , Hino, N. , Aird, W. C. , Doi, T. , & Okada, Y. (2018). Endothelial Robo4 regulates IL‐6 production by endothelial cells and monocytes via a crosstalk mechanism in inflammation. Biochemical and Biophysical Research Communications, 495(1), 801–806. 10.1016/j.bbrc.2017.11.067 [DOI] [PubMed] [Google Scholar]
- Sikora, E. , Bielak‐Zmijewska, A. , Dudkowska, M. , Krzystyniak, A. , Mosieniak, G. , Wesierska, M. , & Wlodarczyk, J. (2021). Cellular senescence in brain aging. Frontiers in Aging Neuroscience, 13, 646924. 10.3389/fnagi.2021.646924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka, T. J. , Whittaker, P. , Sobotka, J. M. , Brodie, R. E. , Quander, D. Y. , Robl, M. , Bryant, M. , & Barton, C. N. (1996). Neurobehavioral dysfunctions associated with dietary iron overload. Physiology & Behavior, 59(2), 213–219. 10.1016/0031-9384(95)02030-6 [DOI] [PubMed] [Google Scholar]
- Spence, H. , McNeil, C. J. , & Waiter, G. D. (2020). The impact of brain iron accumulation on cognition: A systematic review. PLoS One, 15(10), e0240697. 10.1371/journal.pone.0240697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic, S. M. , Martinez‐Revollar, G. , Hu, A. , Choi, J. , Keep, R. F. , & Andjelkovic, A. V. (2019). Decline in Sirtuin‐1 expression and activity plays a critical role in blood‐brain barrier permeability in aging. Neurobiology of Disease, 126, 105–116. 10.1016/j.nbd.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, C. , Riedl, S. , Ruthnick, D. , Notzold, R. R. , & Bauer, U. M. (2012). The arginine methyltransferase PRMT6 regulates cell proliferation and senescence through transcriptional repression of tumor suppressor genes. Nucleic Acids Research, 40(19), 9522–9533. 10.1093/nar/gks767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T. , Warrington, N. M. , Luo, J. , Brooks, M. D. , Dahiya, S. , Snyder, S. C. , Sengupta, R. , & Rubin, J. B. (2014). Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. The Journal of Clinical Investigation, 124(9), 4123–4133. 10.1172/JCI71048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T. , Maekawa, N. , Kashio, T. , Izawa, K. , Ishiba, R. , Shirakura, K. , Ishimoto, K. , Hino, N. , Aird, W. C. , Doi, T. , & Okada, Y. (2017). Tumor necrosis factor alpha induces the expression of the endothelial cell‐specific receptor Roundabout4 through the nuclear factor‐kappaB pathway. Biological & Pharmaceutical Bulletin, 40(4), 504–509. 10.1248/bpb.b16-00938 [DOI] [PubMed] [Google Scholar]
- Tezze, C. , Romanello, V. , Desbats, M. A. , Fadini, G. P. , Albiero, M. , Favaro, G. , Ciciliot, S. , Soriano, M. E. , Morbidoni, V. , Cerqua, C. , Loefler, S. , Kern, H. , Franceschi, C. , Salvioli, S. , Conte, M. , Blaauw, B. , Zampieri, S. , Salviati, L. , Scorrano, L. , & Sandri, M. (2017). Age‐associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metabolism, 25(6), 1374–1389.e1376. 10.1016/j.cmet.2017.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciak, A. R. , Barnes, J. , Ejiogu, N. , Foster, K. , Brant, L. J. , Zonderman, A. B. , & Evans, M. K. (2008). Age, sex, and race influence single‐strand break repair capacity in a human population. Free Radical Biology & Medicine, 45(12), 1631–1641. 10.1016/j.freeradbiomed.2008.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, W. Z. , Li, B. , Huang, B. , Wang, Y. , Liu, X. D. , Guan, H. , Zhang, S. M. , Tang, Y. , Rang, W. Q. , & Zhou, P. K. (2013). gammaH2AX foci formation in the absence of DNA damage: Mitotic H2AX phosphorylation is mediated by the DNA‐PKcs/CHK2 pathway. FEBS Letters, 587(21), 3437–3443. 10.1016/j.febslet.2013.08.028 [DOI] [PubMed] [Google Scholar]
- Uberti, V. H. , de Freitas, B. S. , Molz, P. , Bromberg, E. , & Schroder, N. (2020). Iron overload impairs autophagy: Effects of rapamycin in ameliorating iron‐related memory deficits. Molecular Neurobiology, 57(2), 1044–1054. 10.1007/s12035-019-01794-4 [DOI] [PubMed] [Google Scholar]
- Ueno, M. , Chiba, Y. , Matsumoto, K. , Murakami, R. , Fujihara, R. , Kawauchi, M. , Miyanaka, H. , & Nakagawa, T. (2016). Blood‐brain barrier damage in vascular dementia. Neuropathology, 36(2), 115–124. 10.1111/neup.12262 [DOI] [PubMed] [Google Scholar]
- Walker, A. E. , Morgan, R. G. , Ives, S. J. , Cawthon, R. M. , Andtbacka, R. H. , Noyes, D. , Lesniewski, L. A. , Richardson, R. S. , & Donato, A. J. (2016). Age‐related arterial telomere uncapping and senescence is greater in women compared with men. Experimental Gerontology, 73, 65–71. 10.1016/j.exger.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C. , Wang, L. , Fozouni, P. , Evjen, G. , Chandra, V. , Jiang, J. , Lu, C. , Nicastri, M. , Bretz, C. , Winkler, J. D. , Amaravadi, R. , Garcia, B. A. , Adams, P. D. , Ott, M. , Tong, W. , Johansen, T. , Dou, Z. , & Berger, S. L. (2020). SIRT1 is downregulated by autophagy in senescence and ageing. Nature Cell Biology, 22(10), 1170–1179. 10.1038/s41556-020-00579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, M. , Hayashi, M. , Sonohara, F. , Yamada, S. , Tanaka, H. , Sakai, A. , Mii, S. , Kobayashi, D. , Kurimoto, K. , Tanaka, N. , Inokawa, Y. , Takami, H. , Hattori, N. , Kanda, M. , Tanaka, C. , Nakayama, G. , Koike, M. , & Kodera, Y. (2022). Downregulation of ROBO4 in pancreatic cancer serves as a biomarker of poor prognosis and indicates increased cell motility and proliferation through activation of MMP‐9. Annals of Surgical Oncology, 29(11), 7180–7189. 10.1245/s10434-022-12039-5 [DOI] [PubMed] [Google Scholar]
- Yamazaki, Y. , Baker, D. J. , Tachibana, M. , Liu, C. C. , van Deursen, J. M. , Brott, T. G. , Bu, G. , & Kanekiyo, T. (2016). Vascular cell senescence contributes to blood‐brain barrier breakdown. Stroke, 47(4), 1068–1077. 10.1161/STROKEAHA.115.010835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, H. F. , Zou, T. , Tuo, Q. Z. , Xu, S. , Li, H. , Belaidi, A. A. , & Lei, P. (2021). Ferroptosis: Mechanisms and links with diseases. Signal Transduction and Targeted Therapy, 6(1), 49. 10.1038/s41392-020-00428-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Yang, L. , Li, Y. , Yan, G. , Feng, C. , Liu, T. , Gong, R. , Yuan, Y. , Wang, N. , Idiiatullina, E. , Bikkuzin, T. , Pavlov, V. , Li, Y. , Dong, C. , Wang, D. , Cao, Y. , Han, Z. , Zhang, L. , Huang, Q. , … Cai, B. (2017). Melatonin protects bone marrow mesenchymal stem cells against iron overload‐induced aberrant differentiation and senescence. Journal of Pineal Research, 63(3), e12422. 10.1111/jpi.12422 [DOI] [PubMed] [Google Scholar]
- Yang, S. , Mi, X. , Chen, Y. , Feng, C. , Hou, Z. , Hui, R. , & Zhang, W. (2018). MicroRNA‐216a induces endothelial senescence and inflammation via Smad3/IkappaBalpha pathway. Journal of Cellular and Molecular Medicine, 22(5), 2739–2749. 10.1111/jcmm.13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , Sun, Y. , Lu, Z. , Leak, R. K. , & Zhang, F. (2017). The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Research Reviews, 34, 15–29. 10.1016/j.arr.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Huang, C. , Wu, Y. , Chen, B. , Zhang, W. , & Zhang, J. (2019). Autophagy protects the blood‐brain barrier through regulating the dynamic of Claudin‐5 in short‐term starvation. Frontiers in Physiology, 10, 2. 10.3389/fphys.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Lin, P. , Chen, B. , Zhang, X. , Xiao, W. , Wu, S. , Huang, C. , Feng, D. , Zhang, W. , & Zhang, J. (2021). Autophagy alleviates hypoxia‐induced blood‐brain barrier injury via regulation of CLDN5 (claudin 5). Autophagy, 17(10), 3048–3067. 10.1080/15548627.2020.1851897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, W. C. , Chen, H. J. , Leong, K. H. , Chang, K. L. , Wang, Y. T. , Wu, L. C. , Tung, P. Y. , Kuo, C. F. , Lin, C. C. , & Tsai, S. Y. (2021). The risk of fibromyalgia in patients with iron deficiency anemia: A nationwide population‐based cohort study. Scientific Reports, 11(1), 10496. 10.1038/s41598-021-89842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, M. K. , Kang, S. H. , Jeong, K. B. , Lee, H. S. , Jeon, H. J. , Eun, H. S. , Lee, E. S. , Moon, H. S. , Kim, S. H. , Sung, J. K. , Lee, B. S. , & Jeong, H. Y. (2022). Comparison of gene expression profiles of signet ring cell carcinoma and poorly cohesive carcinoma in early gastric cancer. Clin Med Insights Oncol, 16, 11795549221097941. 10.1177/11795549221097941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, L. H. , Li, F. , Wang, L. , Zhao, S. E. , Wang, S. M. , Zhang, L. L. , Zhang, L. H. , Duan, X. L. , Yu, P. , & Chang, Y. Z. (2015). Brain iron accumulation exacerbates the pathogenesis of MPTP‐induced Parkinson's disease. Neuroscience, 284, 234–246. 10.1016/j.neuroscience.2014.09.071 [DOI] [PubMed] [Google Scholar]
- Yousefzadeh, M. , Henpita, C. , Vyas, R. , Soto‐Palma, C. , Robbins, P. , & Niedernhofer, L. (2021). DNA damage‐how and why we age? eLife, 10, e62852. 10.7554/eLife.62852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh, M. J. , Zhao, J. , Bukata, C. , Wade, E. A. , McGowan, S. J. , Angelini, L. A. , Bank, M. P. , Gurkar, A. U. , McGuckian, C. A. , Calubag, M. F. , Kato, J. I. , Burd, C. E. , Robbins, P. D. , & Niedernhofer, L. J. (2020). Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell, 19(3), e13094. 10.1111/acel.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, P. , Li, Y. , Zhong, G. , Li, W. , Chen, B. , & Zhang, J. (2021). Claudin‐5 affects endothelial autophagy in response to early hypoxia. Frontiers in Physiology, 12, 737474. 10.3389/fphys.2021.737474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, L. J. , Xiu, J. , Chen, J. , Xie, M. , Gen, L. , & Gao, R. (2017). Enhanced oral bioavailability and tissue distribution of ferric citrate through liposomal encapsulation. CyTA ‐ Journal of Food, 15(1), 7. 10.1080/19476337.2016.1221858 [DOI] [Google Scholar]
- Yuan, P. , Qi, X. , Song, A. , Ma, M. , Zhang, X. , Lu, C. , Bian, M. , Lian, N. , He, J. , Zheng, S. , & Jin, H. (2021). LncRNA MAYA promotes iron overload and hepatocyte senescence through inhibition of YAP in non‐alcoholic fatty liver disease. Journal of Cellular and Molecular Medicine, 25(15), 7354–7366. 10.1111/jcmm.16764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Li, L. , Wang, Z. , Liu, H. , Hou, J. , Zhang, M. , Hao, A. , Liu, Y. , He, G. , Shi, Y. , He, L. , Wang, X. , Wan, Y. , & Li, B. (2013). A role for c‐Abl in cell senescence and spontaneous immortalization. Age (Dordrecht, Netherlands), 35(4), 1251–1262. 10.1007/s11357-012-9452-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Yang, D. , Cheng, H. S. , McCoy, M. G. , Pérez‐Cremades, D. , Haemmig, S. , Wong, D. , Chen, L. , & Feinberg, M. W. (2022). miR‐181b regulates vascular endothelial aging by modulating an MAP3K3 signaling pathway. The FASEB Journal, 36(6), e22353. 10.1096/fj.202200046R [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1
FigureS2
FigureS3
FigureS4
FigureS5
FigureS6
FigureS7
FigureS8
TableS1
Data Availability Statement
The data that support the findings of this study are openly available in Harvard Dataverse at https://doi.org/10.7910/DVN/B9YKGC.


