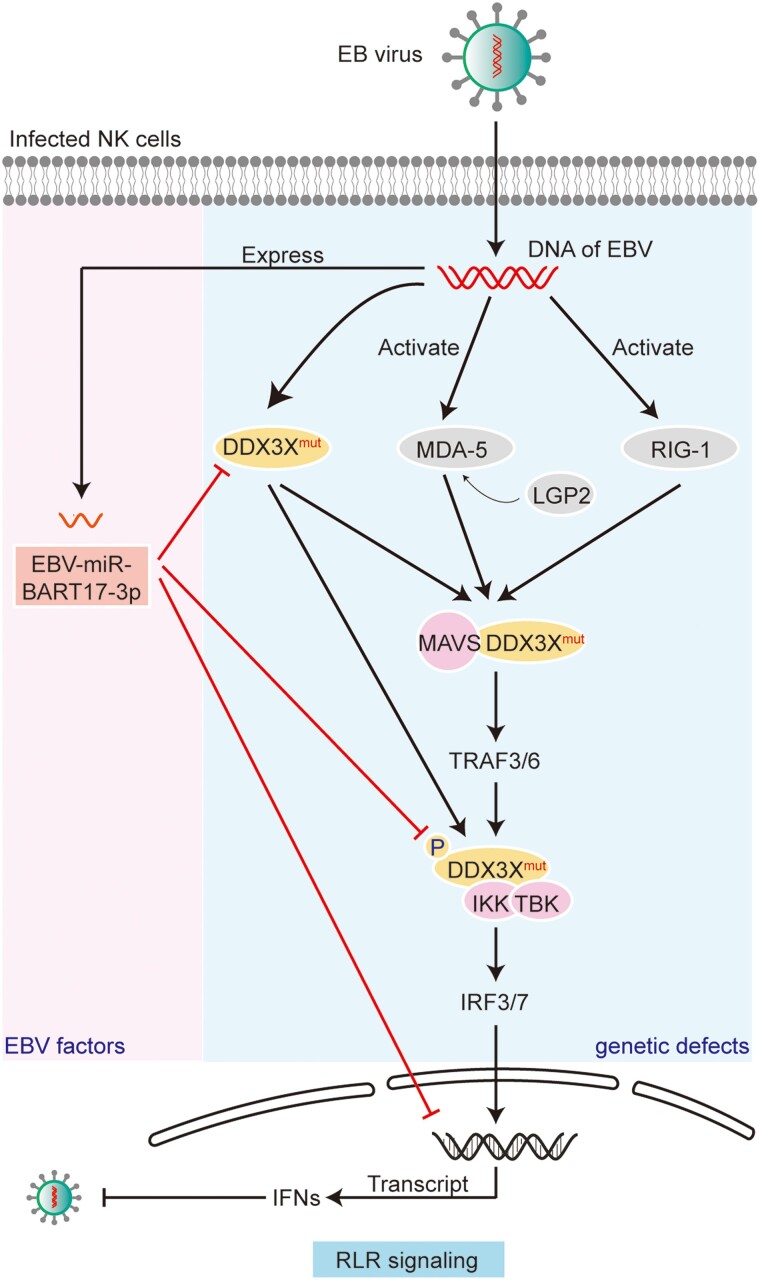
Figure 5.
The proposed mechanism of persistent EBV infection in NK cells. Normally, RLR family members RIG-1 and MDA5, supported by LGP2, are able to sense the viral RNAs once NK cells are infected by EBV. After activation, RLRs interact with MAVS to recruit downstream signaling molecules (IKKε, TBK1, and IKKα/β) and activate IRF-3/7, which leads to IFN secretion, thereby enhancing the antiviral immune response. Defects in the host DDX3X gene negatively affect multiple molecules of RLR signaling (blue background), including its interactions with MAVS and TBK1, thus downregulating the secretion of IFNs. In addition, EBV can further inhibit the RLR antiviral pathway by expressing EBV-miR-BART17-3p targeting DDX3X (pink background). In conclusion, the genetic defects and viral factors in the RLR pathway might synergistically result in impaired immune function and persistent EBV infection in NK cells. EBV, Epstein-Barr virus; IFN, interferon; IKK, inhibitor of NF-κB kinase; IRF3/7, IFN regulatory factor 3/7; LGP2, laboratory of genetics and physiology 2; MAVS, mitochondrial antiviral signaling; MDA-5 (also IFIH1), melanoma differentiation–associated gene 5; NK, natural killer; RIG-1, retinoic acid–inducible gene 1; RLR, RIG-I–like receptors; TBK, TANK-binding kinase; TRAF3/6, TNF receptor–associated factor 3/6.