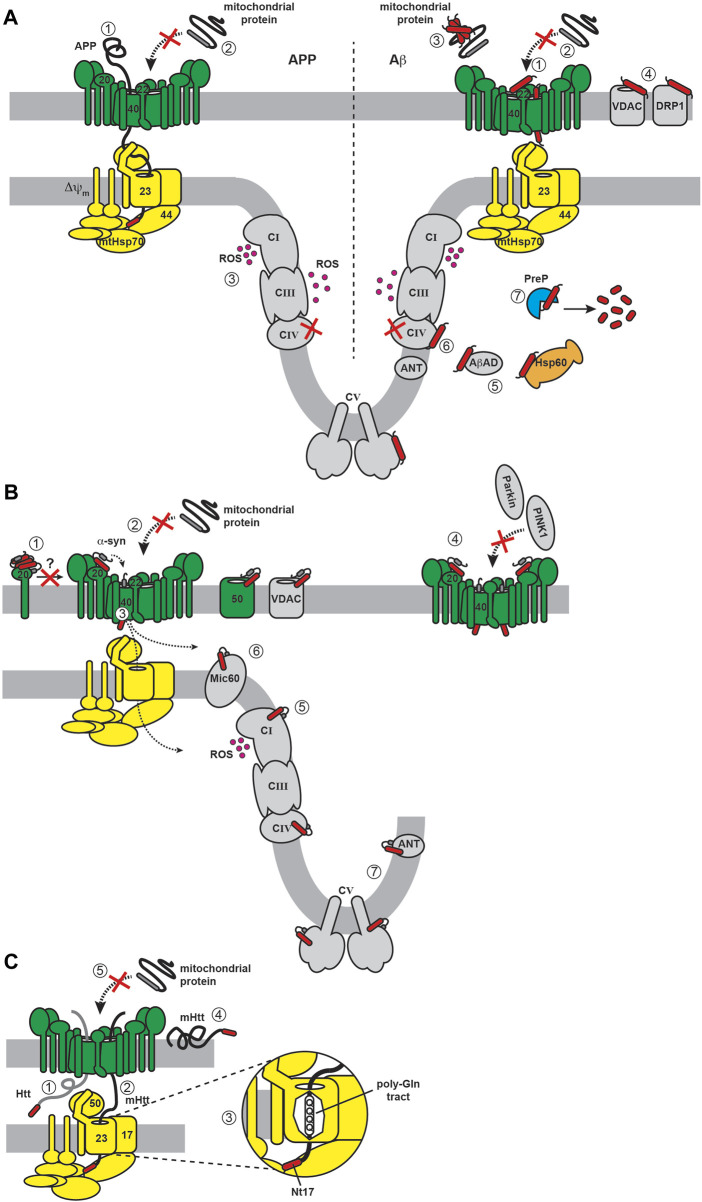
FIGURE 10.
Interactions of amyloidogenic proteins with the TIM23 complex. Models show import complex color scheme used in Figure 2 (TOM/SAM complex, green; TIM23 complex, yellow) with other proteins depicted in grey. Subunits known to interact with amyloids are explicitly indicated by their names. Putative cryptic targeting signals of Aβ/APP, α-syn, and Htt/mHtt are shown in red. [(A), left] APP. (1) APP forms a two membrane-spanning intermediate, engaging both TOM and TIM23 complexes in a Δψm-dependent manner, with complete translocation blocked by the tight folding of the C-terminal AICD domain. (2) Stalled APP intermediates block the import of native mitochondrial precursor proteins. (A, right) Aβ. (1) Aβ interacts with the TOM complex (subunits Tom22 and Tom40), which (2) disrupts the import of native mitochondrial precursors. (3) Aβ forms co-aggregates with mitochondrial precursors in the cytosol. Aβ interacts with proteins of the (4) MOM, (5) matrix, and (6) MIM, most notably CIV and CV. (7) Aβ is degraded by the matrix PreP protease. A general feature of APP/Aβ stress is dysfunctional CIV and excess ROS production by the OXPHOS machinery. (B) α-syn. (1) Multimers of α-syn interact with free Tom20, blocking its assembly with the TOM complex. The engagement of α-syn with TOM complex receptors and channel may (2) hinder import of native mitochondrial proteins, (3) reduce Tom40 expression, and/or (4) disrupt PINK1/Parkin-mediated mitophagy; (5) Interaction of α-syn with CI or CIV may disrupt ETC activity and cause ROS overproduction; (6) Interaction of α-syn with MICOS subunit Mic60 may affect cristae morphogenesis; (7) Interaction of α-syn with ATP synthase (CV) or the adenine nucleotide translocase (ANT) may disrupt adenine nucleotide flux and/or ATP production. (C) Htt/mHtt. (1) Htt may engage the TOM machinery with a tendency to accumulate in the IMS. (2) mHtt engages the TIM23 complex, (3) possibly facilitated by the unstructured poly-Q segment C-terminal to Nt17, which provides a flexible linker for Nt17 to engage TIM23 subunits. (4) mHtt may alternatively accumulate on the cytosolic side of the MOM. (5) Blockage of the import machinery by Htt/mHtt hinders import of mitochondrial precursors.