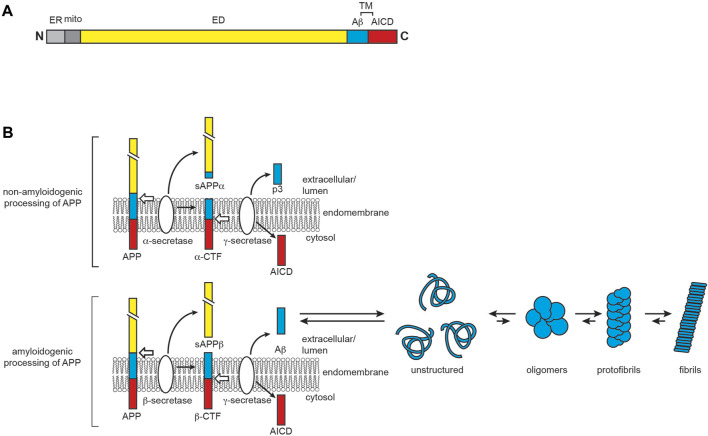
FIGURE 6.
AD and the role of APP and Aβ. (A) APP domain organization. Full-length APP contains an extracellular domain (ED, yellow), a transmembrane region (TM), and the APP intracellular domain (AICD, red). The proteolysis product corresponding to Aβ is shown in cyan. The N-terminal ER- and mitochondria-targeting sequences are shown in light and dark gray, respectively. (B) APP processing and Aβ aggregation. APP has two main proteolytic fates. In the non-amyloidogenic pathway (top), APP is first cleaved by α-secretase to yield soluble APPα (sAPPα) and the α-C-terminal fragment (α-CTF), the latter of which is then cleaved by γ-secretase to produce AICD and the non-amyloidogenic extracellular peptide p3. In the amyloidogenic pathway (bottom), β-secretase proteolyzes full-length APP to yield soluble APPβ (sAPPβ) and the β-C-terminal fragment (β-CTF), the latter of which is then cleaved by γ-secretase to produce AICD and the amyloid beta peptide Aβ. The Aβ peptide, particularly Aβ42, is disordered in solution and prone to aggregation and fibrillization.