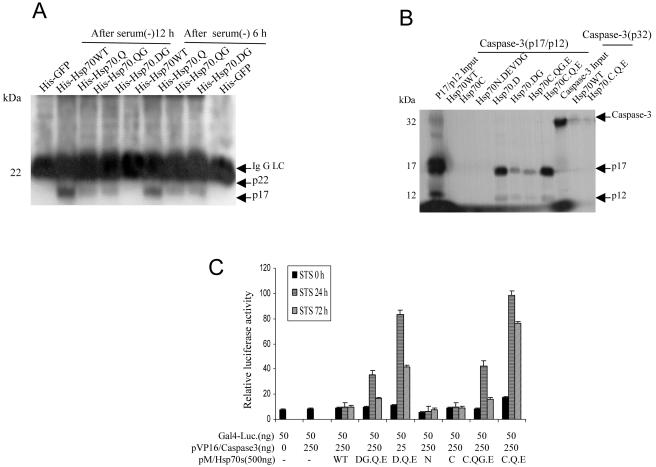
Fig 3.
Hsp70 mutants interact with active caspase-3. (A) NT cells were cultured in serum-free conditions for 6 hours and then returned to 10% serum in Dulbecco's modified Eagle's medium (DMEM) + F12 medium for an additional 6 and 12 hours. The cells were treated with 5 μM protein transduction domain (PTD)–Hsp70 or its mutants for 30 minutes. Cells were resuspended in lysis buffer. Equal amounts of protein from cell lysates were immunoprecipitated with histidine (His) antibody and then immunoblotted with an anti–caspase-3 antibody that recognized the active 17 kDa (p17) subunit of caspase-3. His-GFP served as a negative control. (B) Direct interaction of Hsp70 mutants with caspase-3 p17/12 active subunits. Beads pull-down experiments were performed as described in Materials and Methods. The signal generated by 10% of the total input amount of each 35S-labeled protein is shown. (C) In vivo analysis of the interaction of Hsp70 mutants and active caspase-3. The mammalian 2-hybrid assays were performed using the indicated plasmids that were cotransfected into Cos-1 cells, together with the Gal4-dependent luciferase reporter. For each transfection, cells were also transfected with a constant amount of pSv/β-gal for monitoring transfection efficiency and normalization of the luciferase activity. Cells were treated with staurosporine (STS) for 0, 24, or 72 hours