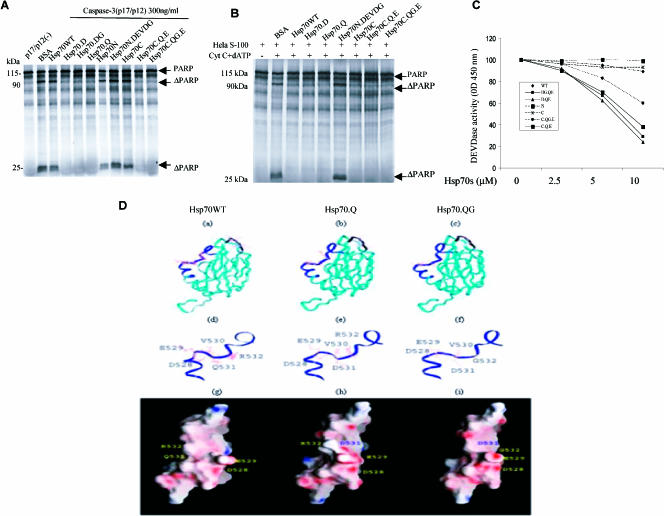
Fig 5.
Inhibitory effects of Hsp70 and its mutants on caspase-3 activity. (A) 35S-labeled PARP was incubated with recombinant active caspase-3 either with or without 15 μM PTD–wild-type Hsp70 protein (Hsp70WT) or its mutant proteins. (B) The Hela S-100 fraction, cytochrome c, and deoxyadenosine triphosphate (dATP) were incubated with recombinant Hsp70WT and its mutants (15 μM) along with 35S-labeled PARP. (C) Inhibition of DEVDase by Hsp70s. The tetrapeptide DEVD-pN (A) was used as a substrate. Fifteen nanograms of active caspase-3 and purified Hsp70WT and its mutants were added at concentrations ranging from 0 to 10 μM for the DEVD-pN assay. (D) Hsp70 protein models for Hsp70.WT (a, d, g), Hsp70.Q (b, e, h), and Hsp70.QG (c, f, i) are shown with amino acids 528–531 represented as a black ribbon in Hsp70.WT (a) and amino acids 521–540 represented as a blue ribbon in Hsp70.Q (b) and Hsp70.QG (c). The detailed models of these amino acid regions (521–540) are also shown for each protein (d, e, f) along with a representation of the surface electrostatic charge for each protein (g, h, i)