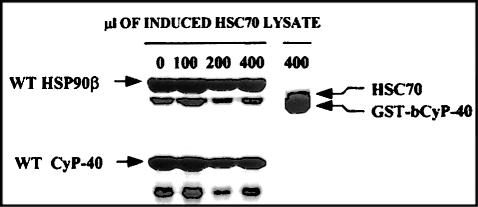
Fig 6.
Determination of a mutually exclusive interaction of heat shock protein (Hsp)90 and heat shock cognate (Hsc)70 with cyclophilin 40 (CyP40). Ni-NTA agarose was charged with His-tagged Hsp90β and mixed 1:1 with Sepharose 4B. Bacterial lysate (150 μL) for wild-type bCyP40 was added to the diluted gel (40 μL), and after dilution to 500 μL total volume with binding buffer containing 35 mM imidazole and 0.2% Triton X-100, the mixture was rotated at 4°C for 3 hours. After removal of unbound proteins by successive washing, the gel containing Hsp90-bound CyP40 was exposed to 0, 100, 200, and 400 μL of induced Hsc70 bacterial lysate. Reaction volumes were brought to 500 μL with binding buffer containing imidazole and Triton X-100, and incubation was continued with rotation at 4°C for 3 hours. Gel-retained proteins were recovered with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by SDS-PAGE with Coomassie blue staining. Glutathione-agarose containing immobilized GST-bCyP40 was mixed 1:5 with Sepharose 4B. The diluted gel (40 μL) was incubated as described above with 400 μL of Hsc70 lysate and gel-retained proteins were analyzed by SDS-PAGE. GST, glutathione S-transferase