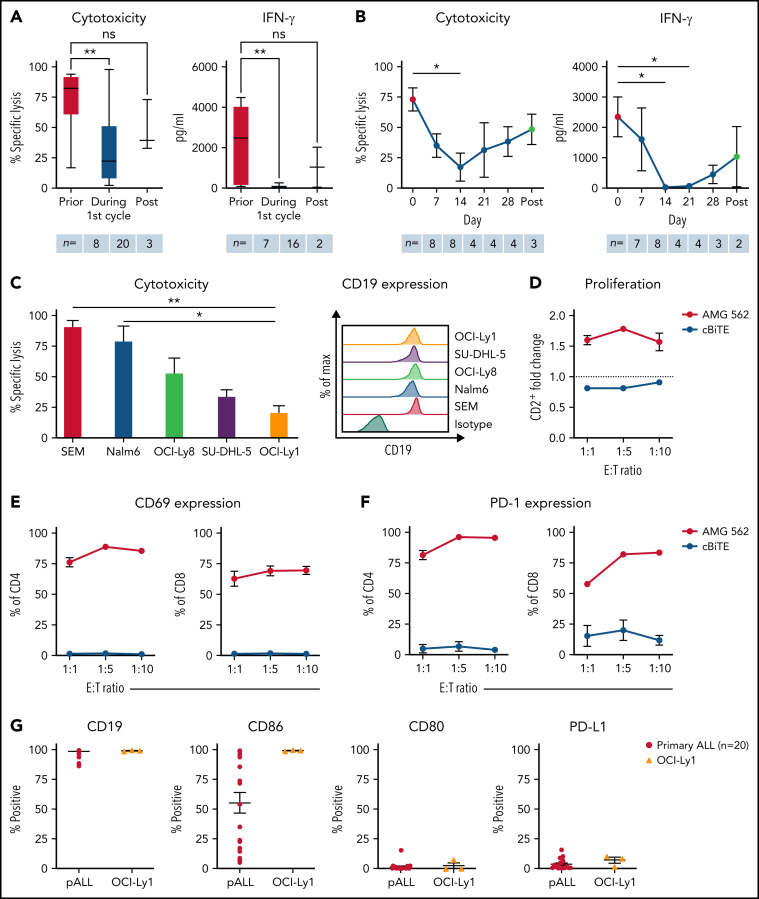
Figure 1.
T cells of blinatumomab-treated ALL patients show signs of exhaustion ex vivo. (A) Blinatumomab-mediated cytotoxicity (n = 8–20) on day 3 and IFN-γ secretion (n = 7–16) on day 6 of T cells against REH cells (blinatumomab/control BiTE = 0.5 ng/mL, E:T = 1:3). The T cells were isolated from ALL patients prior to (“pre”), during and after the first cycle of blinatumomab therapy. (B) Blinatumomab-mediated cytotoxicity (n = 3–8) on day 3 and IFN-γ secretion (n = 2–8) on day 6 of T cells against REH cells (blinatumomab/control BiTE = 0.5 ng/mL, E:T = 1:3). The T cells were isolated from ALL patients at different timepoints during the first cycle of blinatumomab therapy. (C) AMG 562-mediated cytotoxicity (n = 3–6) and of HD T cells against ALL (SEM, Nalm6) and diffuse large B cell lymphoma (OCI-Ly8, SU-DHL-5, OCI-Ly1) cell lines (supplemental Table 1) after 4 days (AMG 562/control BiTE = 5 ng/mL, E:T = 1:3). Representative histograms of CD19 expression on ALL and diffuse large B cell lymphoma cell lines are shown. (D) CD2+ fold change (n = 3) of HD T cells and (E-F) percentage of CD69+ and PD-1+ among CD4+ and CD8+ T cells after 3 days of cytotoxicity assay (AMG 562/control BiTE = 5 ng/mL) against OCI-Ly1 cells; n = 3. (G) Percentage of CD19+, CD86+, CD80+, and PD-L1+ primary ALL (n = 20) and OCI-Ly1 cells (n = 3). Boxplot whiskers indicate minima and maxima, and boxes represent the lower quartile, the median, and the upper quartile. All other graphs present mean ± SEM values. Statistical analysis: Kruskal–Wallis and Dunn's multiple comparison test (A–C); nsP > .05; *P < .05; **P < .01. ALL, acute lymphoblastic leukemia; cBiTE, control BiTE, bispecific control construct; E:T, effector/target ratio; HD, healthy donor; ns, not significant; pALL, primary ALL; ± SEM, standard error of the mean.