Figure 2: Cyclin-dependent kinase 12 (CDK12) and serine/threonine protein kinase SMG1 regulate phosphorylation of p63 in human keratinocytes following UV irradiation.
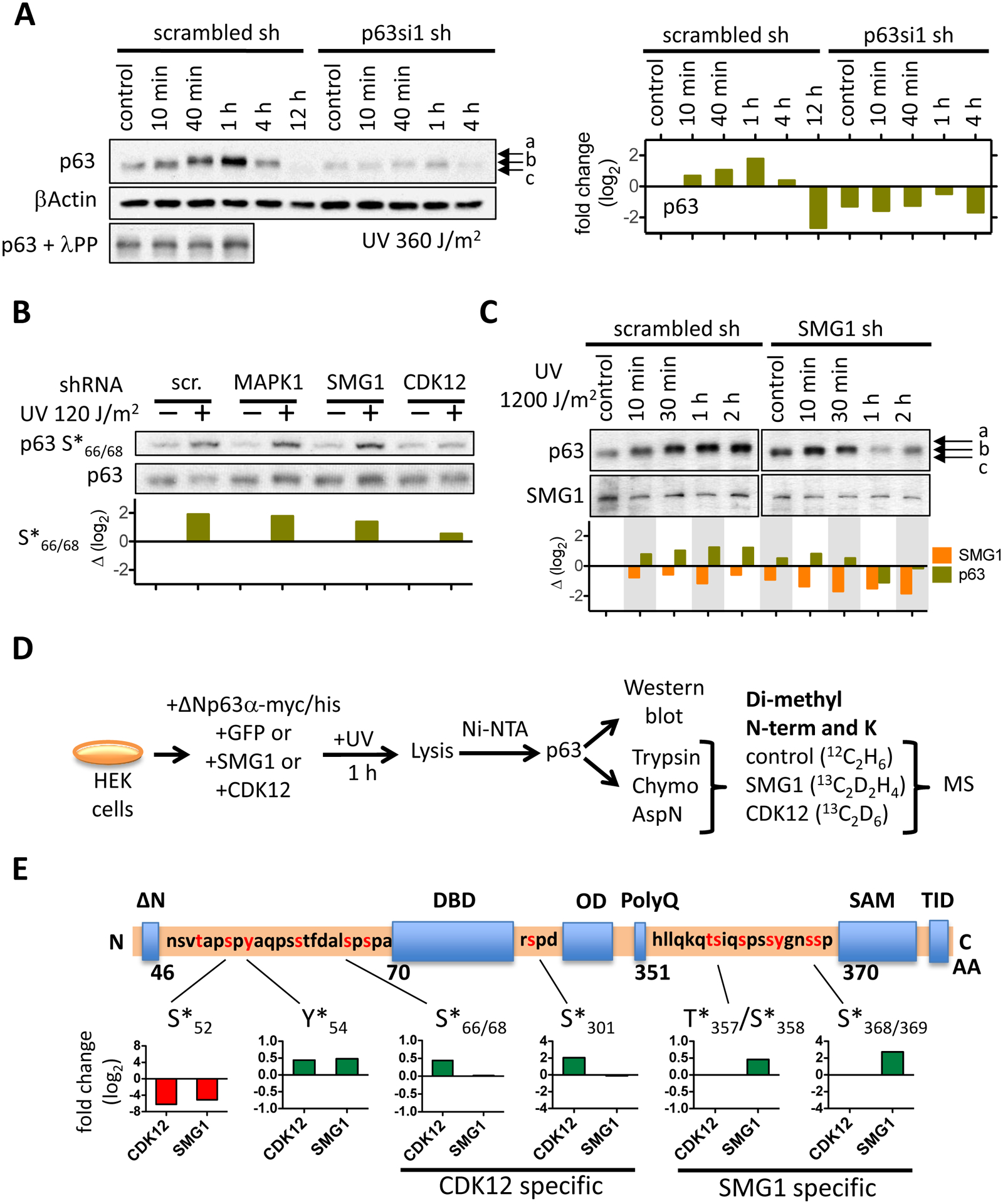
(A) The Western blot shows ΔNp63α phosphorylation following UV irradiation with 360 J/m2 in a time-course experiment. An aliquot of control and of each of the first three time points were digested with λ protein phosphatase to show that the electrophoretic shift of p63 is dependent on phosphorylation. Relative ΔNp63α protein levels are depicted in the bar graph (right). (B) MAPK1 or SMG1 or CDK12 was depleted in NHEK with specific shRNAs, NHEK UV irradiated (120 J/m2), ΔNp63α phosphorylation at S66/68 detected by Western blot. The bar graph depicts the fold change of ΔNp63α phosphorylation in the kinase knock-down cells relative to control. (C) ΔNp63α protein levels and its SDS-gel electrophoretic migration pattern was determined in NHEK following SMG1 knock-down and UV irradiation. Relative protein levels are indicated in the bar graph below the Western blot. (D) The schematic depicts the detection of ΔNp63α phosphorylation with mass spectrometry. ΔNp63α was transiently overexpressed in human embryonic kidney cells (HEK293) and phosphorylation in presence of either CDK12 or SMG1 quantified following digest with different proteolytic enzymes, isotope labeling of peptides by reductive methylation and quantitative mass spectrometry. (E) Phosphorylation of ΔNp63α by SMG1 and CDK12 is localized within the ΔNp63α protein. The relative change in phosphorylation at specific sites is normalized to the phosphorylation level of ΔNp63α in control HEK293 cells that were transfected with GFP. The bar graphs depict the relative change in phosphorylation at the amino acid sites that are indicated.
