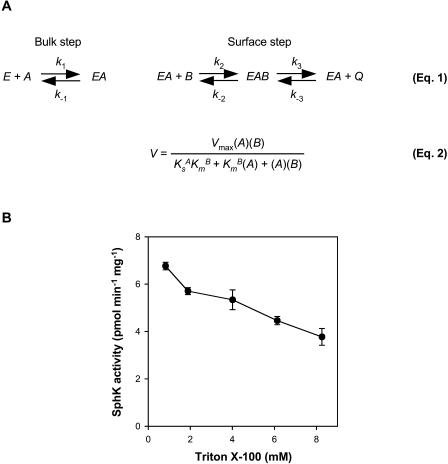
Figure 3.
Principle of the surface dilution model. A, The principle of the surface dilution model is presented in Equation 1 (Carman et al., 1995). According to this model, the action of the enzyme E consists of two consecutive steps. First, the enzyme interacts noncatalytically with the surface of detergent/lipid mixed micelles A. Subsequently, the enzyme mixed-micelle complex EA binds to the individual lipid substrate B presented on the surface, which leads to the conversion of substrate to product Q. The first association depends on the bulk concentration of both E and A, whereas the second step depends on the surface concentrations of EA and B. A is defined as the sum of the molar concentrations of detergent plus lipid substrate, and B is the mole fraction of lipid substrate in the mixed micelle. Equation 2 is the rate expression for the surface dilution kinetic model (Carman et al., 1995). Three kinetic parameters, Vmax, KsA, and KmB, can be determined from this equation. Vmax is the true Vmax when both the bulk concentration and the surface concentration of the lipid substrate approach infinity. KsA, which equals k−1/k1 and is expressed in bulk concentration terms, is the dissociation constant describing the interaction of the enzyme with the mixed micelles in the first binding step. KmB, equal to (k−2 + k3)/k2, defines the interfacial Km for the second binding step and is expressed in surface concentration units, such as mole fraction. B, SphK activity in leaf lysates measured with increasing Triton X-100 molar concentrations in the mixed micelles. The molar concentration of Sph was held constant at 50 μm. Data are means ± se of five independent experiments.