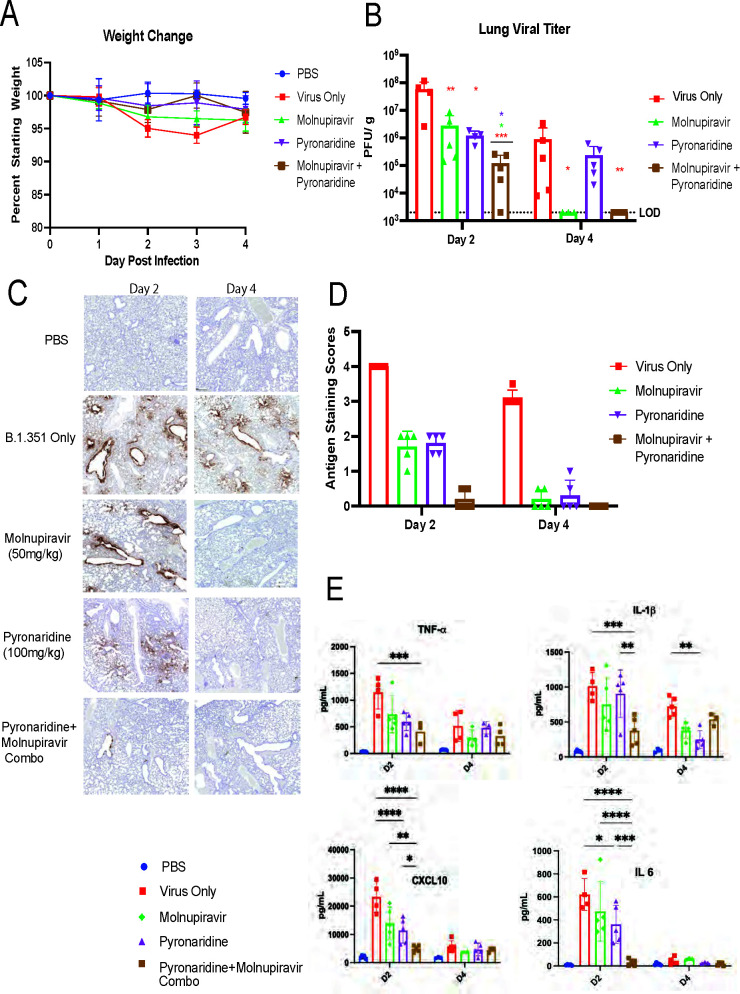
Fig 2.
Combination of molnupiravir and pyronaridine treatment synergistically reduces SARS-CoV-2 infection and inflammation in vivo compared to single drug treatment. Eight- to 10-week-old wild-type BALB/c mice (n = 5 per group) were challenged with 1 × 105 PFU of SARS-CoV-2 Beta variant (B.1.351) and treated with either nothing, molnupiravir, pyronaridine, or combination treatment of molnupiravir and pyronaridine orally beginning 12 h before infection. (A) Mouse weights were measured each day and lungs were harvested at 2 dpi for (B) mouse lungs that were analyzed for viral titer quantification by plaque assay on day 2 and day 4. (C) Lungs were imaged using nucleocapsid staining via IHC antibody on the lungs on day 2 and day 4 as well as by H&E staining for inflammatory pathology (Fig. S1). (D) Nucleocapsid staining was scored by percentage stained. (E) Lungs were harvested and analyzed for protein levels using a Bio-Plex Pro Mouse Chemokine Panel, a complete heat map of the chemokine/cytokine panel is available (Fig. S1). n = 5 mice per group, mean ± SD is shown. *P < 0.05, **P < 0.01, and ***P < 0.001, lung titers were analyzed for significance by log transformation and mixed-effect analysis with the Tukey test for multiple comparisons. Cytokine comparisons were analyzed by mixed-effect analysis followed by the Sidak test for multiple comparisons. The red asterisks are compared to virus-only group, whereas the green and purple asterisks are compared to molnupiravir and pyronaridine groups, respectively.