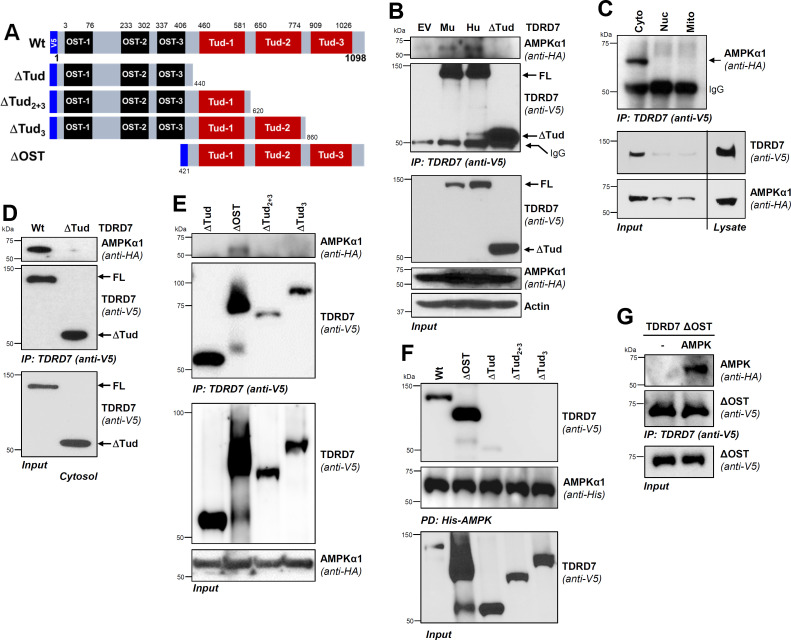
Fig 2.
The Tudor-3 domain of TDRD7 is required for interaction with AMPK. (A) Wild-type (Wt) TDRD7 with its functional domains (OST and Tud) and the deletion mutants used in the study. (B) HEK293T cells were co-transfected with V5-TDRD7 (Hu, human; Mu, mouse), V5-Tdrd7 (mouse), or Tud-deleted mutant (ΔTud) and HA-AMPKα1 plasmids. TDRD7 was immunoprecipitated from the cell lysates, and the immunoprecipitates were analyzed for AMPK by immunoblot. (C) HEK293T cells were co-transfected with V5-TDRD7 and HA-AMPKα1 plasmids. TDRD7 was immunoprecipitated from cytosolic, mitochondrial, and nuclear fractions, and the immunoprecipitates were analyzed for AMPK by immunoblot. (D) HEK293T cells were co-transfected with Wt TDRD7 or the Tudor-deleted mutant (ΔTud) and HA-AMPKα1 plasmids; TDRD7 was immunoprecipitated from cytosolic fractions. The immunoprecipitates were analyzed for AMPK by immunoblot. (E) HEK293T cells were co-transfected with V5-TDRD7 deletion mutants and HA-AMPKα1 plasmids, as indicated. TDRD7 was immunoprecipitated from cell lysates, and the immunoprecipitates were analyzed for AMPK by immunoblot. (F) HEK293T cells were co-transfected with Wt V5-TDRD7 or the deletion mutants (as indicated) and His-AMPK plasmids; AMPK was pulled down by Ni-NTA-agarose from the cell lysates and analyzed for TDRD7 by immunoblot. (G) TDRD7 ΔOST mutant and AMPK were expressed bacterially; the recombinant proteins were used for in vitro interaction followed by co-immunoprecipitation, as indicated. FL, full length. The results are representative of at least three experiments.