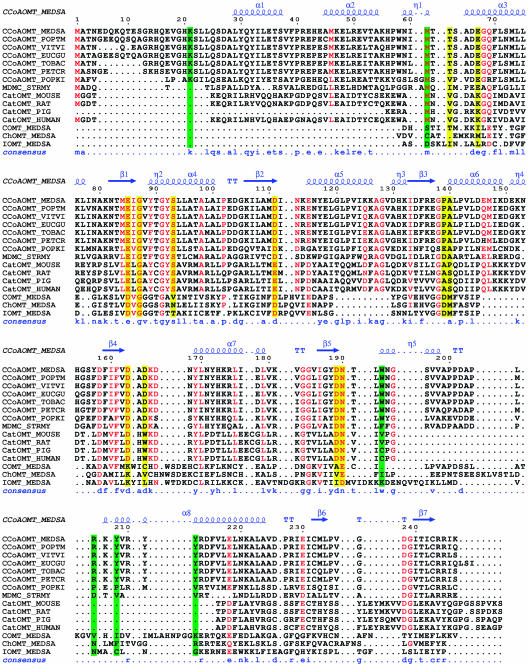
Figure 2.
Sequence alignment of OMTs. Sequence alignment of alfalfa CCoAOMT with related OMTs, including 6 plant OMTs that exhibit a preference for CoA-linked thioesters, one macrolide 4-OMT (MDMC_STRMY or Ma40MT) from Streptomyces mycarofaciens, the soluble part of 4 animal catechol OMTs, including the structurally characterized rat catechol OMT (Protein Data Bank accession no. 1VID), and 3 structurally characterized plant OMTs (alfalfa COMT, ChOMT, and IOMT; Protein Data Bank accession nos. 1KYZ, 1FPQ, and 1FPX, respectively) truncated to the region that aligns with CCoAOMT. The secondary structure of alfalfa CCoAOMT is displayed above and the consensus sequence for the CCoAOMT family below the alignment. Highly conserved residues between CCoAOMT and catechol OMT families are red. Residues involved in divalent metal ion and cofactor binding are depicted on a yellow background. Residues involved in substrate recognition are depicted on a green background. This figure was prepared with ESPript (Gouet et al., 1999) and corrected by hand to closely match structural alignments when such structures are available.