Abstract
Progress is being made in understanding the biochemical and molecular basis of nickel (Ni)/zinc (Zn) hyperaccumulation in Thlaspi; however, the molecular signaling pathways that control these mechanisms are not understood. We observed that elevated concentrations of salicylic acid (SA), a molecule known to be involved in signaling induced pathogen defense responses in plants, is a strong predictor of Ni hyperaccumulation in the six diverse Thlaspi species investigated, including the hyperaccumulators Thlaspi goesingense, Thlaspi rosulare, Thlaspi oxyceras, and Thlaspi caerulescens and the nonaccumulators Thlaspi arvense and Thlaspi perfoliatum. Furthermore, the SA metabolites phenylalanine, cinnamic acid, salicyloyl-glucose, and catechol are also elevated in the hyperaccumulator T. goesingense when compared to the nonaccumulators Arabidopsis (Arabidopsis thaliana) and T. arvense. Elevation of free SA levels in Arabidopsis, both genetically and by exogenous feeding, enhances the specific activity of serine acetyltransferase, leading to elevated glutathione and increased Ni resistance. Such SA-mediated Ni resistance in Arabidopsis phenocopies the glutathione-based Ni tolerance previously observed in Thlaspi, suggesting a biochemical linkage between SA and Ni tolerance in this genus. Intriguingly, the hyperaccumulator T. goesingense also shows enhanced sensitivity to the pathogen powdery mildew (Erysiphe cruciferarum) and fails to induce SA biosynthesis after infection. Nickel hyperaccumulation reverses this pathogen hypersensitivity, suggesting that the interaction between pathogen resistance and Ni tolerance and hyperaccumulation may have played a critical role in the evolution of metal hyperaccumulation in the Thlaspi genus.
Worldwide, more than 400 plant species are now known that hyperaccumulate various trace metals (Cd, Co, Cu, Mn, Ni, and Zn), nonmetals (Se; for review, see Reeves and Baker, 2000), and metalloids (As; Ma et al., 2001) in their shoots when growing in their native habitats. Of these, many are Brassicaceae family members, including numerous Thlaspi species that hyperaccumulate nickel (Ni) and zinc (Zn) up to 3% (30,000 μg g−1) of their shoot dry weight. The extraordinary ability of these plants to hyperaccumulate Ni/Zn make them an ideal source of genetic material for the development of both mineral nutrient-fortified crops and plants suitable for phytoremediation of metal-polluted soils and waters (Guerinot and Salt, 2001). To develop a practical genetic model system for dissecting the mechanistic basis of metal hyperaccumulation, we have been studying numerous biannual Ni and Zn hyperaccumulators from the Brassicaceae, including many members of the Thlaspi genus collected from both serpentine and mine sites in Austria, France, Greece, Turkey, and the United States (Peer et al., 2003). To complement these broad-based studies, we have also focused on Thlaspi goesingense Hálácsy (Brassicaceae), a hyperaccumulator species found growing on naturally Ni-enriched serpentine soils in Redschlag, Austria, where it accumulates up to 1.2% of its shoot dry weight as Ni (Reeves and Brooks, 1983; Krämer et al., 1997; Wenzel and Jockwer, 1999).
Nickel hyperaccumulation in T. goesingense is primarily determined by its high degree of Ni tolerance (Krämer et al., 1997; Salt et al., 1999), achieved through an efficient system to pump and store Ni in the central vacuole of shoot cells (Krämer et al., 2000; Küpper et al., 2001). We observed that a member of the cation diffusion facilitator family, TgMTP1, is constitutively highly expressed in T. goesingense and may be playing a role in vacuolar sequestration of metal in the hyperaccumulator (Persans et al., 2001). Similar constitutively enhanced expression has also been observed for the TgMTP1 homologs ZTP1 and AhMTP1 in the Zn hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri (Assunção et al., 2001; Becher et al., 2004). Furthermore, in crosses between the Zn hyperaccumulator A. halleri and the nonaccumulating relative Arabidopsis lyrata, AhMTP1 overexpression was found to cosegregate with Zn tolerance (Dräger et al., 2004). Intriguingly, recent data from our laboratory suggests that TgMTP1 may also be acting at the plasma membrane as a metal efflux pump (Kim et al., 2004). From our cellular Ni distribution studies (Krämer et al., 2000), it is clear that a substantial amount of cellular Ni also accumulates outside of the vacuole, suggesting the need for a cytoplasmic-based tolerance mechanism. The recent identification of Ni2+ complexed to the high-affinity metal chelate nicotianamine in the Ni/Zn hyperaccumulator T. caerulescens (Vacchina et al., 2003) suggests that nicotianamine may play an important role in detoxification of extravacuolar Ni in hyperaccumulating plants. Constitutive overproduction of nicotianamine and the enzyme responsible for its biosynthesis, nicotianamine synthase, in T. caerulescens and the related hyperaccumulator A. halleri (Vacchina et al., 2003; Becher et al., 2004; Weber et al., 2004) strongly supports such a conclusion, and suggests that nicotianamine overproduction is a general mechanism underlying Ni/Zn hyperaccumulation in the Brassicaceae family. Recently, we observed that glutathione (GSH) concentrations in Thlaspi hyperaccumulators are also constitutively elevated, leading to enhanced tolerance to Ni-induced oxidative stress (Freeman et al., 2004). Further investigations determined that enhanced GSH in the hyperaccumulators is due to the constitutively enhanced activity of Ser acetyltransferase (SAT; Freeman et al., 2004). Such elevated GSH provides one mechanism whereby the hyperaccumulator is able to resist the oxidative damage caused by nonsequestered Ni2+.
Progress is being made in understanding the biochemical and molecular basis of Ni/Zn tolerance in Thlaspi and other hyperaccumulators. However, the molecular signaling pathways that control these mechanisms are not understood. Salicylic acid (SA) is a potent signaling molecule in plants and is well established to be involved in eliciting specific responses to biotic stresses (for review, see Dempsey et al., 1999; Shah, 2003). Furthermore, SA is also known to be involved in abiotic stress signaling, including plant responses to heavy metals. SA pretreatment alleviates Pb- and Hg-induced membrane damage in rice (Oryza sativa; Mishra and Choudhuri, 1999) and Cd toxicity in barley (Hordeum vulgare) and maize (Zea mays) seedlings (Pál et al., 2002; Metwally et al., 2003). Protection from oxidative damage caused by paraquat (Ananieva et al., 2004), heat (Larkindale and Knight, 2002), cold (Janda et al., 1999), NaCl (Tari et al., 2002), and water deficit (Bezrukova et al., 2001) has also been achieved by SA pretreatments. Interestingly, SA has been shown to accumulate in plants in response to various oxidizing stresses, including hydrogen peroxide (León et al., 1995), ozone (Sharma et al., 1996), and heat (Dat et al., 1998), and this correlates with accumulation of the antioxidant GSH and glutathione reductase (GR), the enzyme responsible for maintaining GSH in its reduced form (Dat et al., 1998; Srivastava and Dwivedi, 1998; Knörzer et al., 1999). It has been suggested that SA is directly involved in signaling these antioxidant responses (Larkindale and Knight, 2002), though the signaling mechanisms remain obscure.
However, a large number of the components of the SA-mediated pathogen response (PR) in plants have been identified (for review, see Dong, 2004; Pieterse and Van Loon, 2004). SA is known to activate PR via NPR1 (NONEXPRESSOR OF PR GENES1), a soluble protein localized to both the cytoplasm and the nucleus (Cao et al., 1997; Ryals et al., 1997; Despres et al., 2000). Nuclear localization of NPR1 is required for the induction of PR genes required for pathogen resistance (Kinkema et al., 2000). Relocalization of NPR1 to the nucleus is driven by the conversion of oligomers of NPR1 into monomers, their interconversion being regulated by the reduction of disulfides within the NPR1 oligomer (Mou et al., 2003). In the nucleus, NPR1 interacts with certain Leu zipper transcription factors, including TGA1, to regulate SA-dependant gene expression. Interaction between NPR1 and TGA1 is also regulated by the reduction of a critical disulfide in TGA1 (Despres et al., 2003). At present, it is unclear how SA regulates the reduction of NPR1 and TGA1 (Dong, 2004). Plants containing a mutant NPR1 allele are able to induce SA biosynthesis in response to a pathogen. However, npr1 plants are hypersensitive to pathogens because SA cannot signal gene expression required for a normal PR. NPR1-independant SA signaling is also known to occur during plant responses to various viral pathogens (Kachroo et al., 2000; Takahashi et al., 2002), though the signaling pathway has still to be determined.
Here, we present evidence that the GSH-mediated Ni tolerance mechanism previously observed in Thlaspi hyperaccumulators (Freeman et al., 2004) is signaled by the constitutively elevated levels of SA observed in these Ni/Zn hyperaccumulators. In the four species of Thlaspi hyperaccumulators tested, SA concentrations are constitutively elevated compared to the nonaccumulators, and this is associated with the elevated SAT activity and GSH biosynthesis previously observed in these species (Freeman et al., 2004). We observe that both biochemical and genetic manipulations that increase SA in Arabidopsis (Arabidopsis thaliana) mimic the GSH-related phenotypes of the hyperaccumulating Thlaspi, and these biochemical changes in the nonaccumulator are associated with increased GSH-mediated Ni resistance. Such observations suggest that SA may be one of the regulators involved in coordinating certain key biochemical differences between Ni/Zn hyperaccumulators and nonaccumulator Thlaspi. Furthermore, our observation that SA mediates accumulation of GSH by activation of SAT, in an NPR1-independent manner, provides a novel mechanism for SA to control the NPR1 and TGA1 oxidation/reduction exchange that is critical for SA signaling of PR (Despres et al., 2003; Mou et al., 2003).
RESULTS
SA and Its Metabolites in Hyperaccumulator and Nonaccumulator Plants
SA, a molecule known to be involved in oxidative stress signaling, is accumulated constitutively in shoot tissue of the hyperaccumulator T. goesingense compared to the related nonaccumulators Thlaspi arvense and Arabidopsis (Table I), to levels comparable to those observed in the SA accumulators willow and wintergreen (Raskin et al., 1990). Similar elevated levels of SA were also observed in T. goesingense shoot tissue collected in the field at Redschlag, Austria (data not shown). Furthermore, SA is not observed in roots or xylem exudates, and concentrations are not significantly affected by Ni exposure (data not shown). Importantly, the SA metabolites Phe, cinnamic acid, salicyloyl-Glc, and catechol are also constitutively elevated in the shoot tissue of the hyperaccumulator T. goesingense compared to the nonaccumulators (Table I). A further comparison of shoot SA levels in the Ni hyperaccumulator Thlaspi oxyceras and the Ni/Zn hyperaccumulators Thlaspi rosulare, T. caerulescens (Puy de Wolf), and T. caerulescens (St Félix de Pallières) with the nonaccumulators T. arvense and Thlaspi perfoliatum reveals free SA levels to be constitutively between 7- and 50-fold higher in all the hyperaccumulators examined (Table II). Such evidence supports the conclusion that at least among the Thlaspi hyperaccumulators, elevated SA is associated with Ni hyperaccumulation. Intriguingly, constitutively elevated SA in T. goesingense is also associated with enhanced susceptibility to powdery mildew (Erysiphe cruciferarum Opiz ex L. Junell) when compared to the susceptible Arabidopsis Columbia (Col-0) ecotype (Adam et al., 1999; Fig. 1A). T. goesingense growing side by side with Arabidopsis consistently show severe infections with E. cruciferarum with extensive development of fungal conidia producing a powdery appearance on leaf surfaces (Fig. 1, B–D). This enhanced susceptibility to E. cruciferarum is suppressed by Ni (Fig. 1A), as has previously been observed for infection of the Ni hyperaccumulator Streptanthus polygaloides (Brassicaceae) with Erysiphe polygonii (Boyd et al., 1994). Furthermore, SA levels in T. goesingense are not significantly affected by pathogen infection (Fig. 1A). Investigations are currently under way in our laboratory to determine if T. goesingense lacks a biochemical and molecular response to E. cruciferarum.
Table I.
Quantification of SA and metabolites in hyperaccumulator and nonaccumulator plants
SA, salicyloyl-Glc, and catachol shown as μmol g−1 fresh weight; Phe and cinnamate as nmol g−1 fresh weight. Data represent average (n = 6 individual plants) ± sd.
| T. goesingense | T. arvense | Arabidopsis | |
|---|---|---|---|
| Phe | 54.4 ± 29.1 | 28.0 ± 7.2 | 18.1 ± 6.2 |
| Cinnamic acid | 52.1 ± 11.6 | nda | nd |
| SA | 68.2 ± 11.5 | 0.13 ± 0.06 | 0.16 ± 0.08 |
| Salicyloyl-Glc | 502.8 ± 30.0 | 0.58 ± 0.06 | 0.10 ± 0.02 |
| Catechol | 316.2 ± 66.7 | 6.8 ± 2.2 | nd |
Not detected.
Table II.
SA, Ni, and Zn concentrations in various Thlaspi species
Column 1, Metal concentrations from shoot tissue harvested from plants grown in a growth chamber in soil augmented with either 100 μg g−1 dry weight Ni or Zn (Peer et al., 2003). Column 2, Metal concentrations determined in shoot samples collected from plants growing in their native environment; data for T. caerulescens from Reeves et al. (2001).
| Species
|
Habitat
|
SAa
|
Ni
|
Zn
|
||
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |||
| μmol g−1 fresh weight mean ± sd | μg g−1 dry weight | |||||
| T. arvense | Calcareous | 0.17 ± 0.08 | 0.15 | 1 | 150 | 33 |
| T. perfoliatum | Calcareous | 0.24 ± 0.13 | 100 | 14 | 400 | 44 |
| T. rosulare | Serpentine | 1.64 ± 0.15 | 1,100 | 27,245 | 1,100 | 77 |
| T. oxyceras | Serpentine | 2.29 ± 0.63 | 1,000 | 16,930 | 200 | 77 |
| T. caerulescens (Puy de Wolf) | Serpentine | 5.41 ± 1.13 | 1,600 | 10,610 | 5,100 | 3,426 |
| T. caerulescens (St Félix de Pallières) | Pb/Zn mine | 9.29 ± 0.99 | 1,100 | 1 | 4,200 | 8,762 |
Data represents the mean ± sd of quantifications from three to seven individual plants per species.
Figure 1.
Response of hyperaccumulator and nonaccumulator to powdery mildew. A, Both Arabidopsis and T. goesingense were cocultivated for 35 d and allowed to become naturally inoculated with powdery mildew. During the growth period, plants were watered twice weekly with one-tenth Hoagland, except one group of T. goesingense plants received one-tenth Hoagland containing 200 μm Ni(NO3)2. Shoot tissue was harvested from all plants and analyzed for SA. Data represent mean μmol g−1 fresh weight SA (n = 8) ± sd. Infected T. goesingense leaves were examined for identification of powdery mildew. B, 1×; C, 6.3×; and D, 100× magnification.
The Role of SA in Ni Tolerance
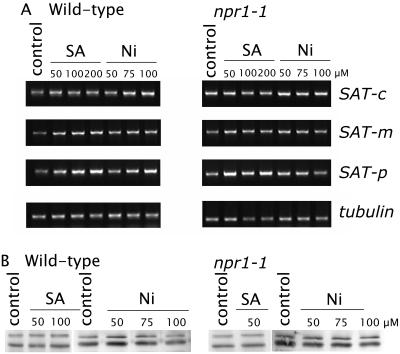
To establish a role for SA in Ni/Zn hyperaccumulation, we performed experiments on the related nonaccumulator Arabidopsis. Pretreatment of Arabidopsis with SA for 14 d, prior to Ni exposure for 8 d, causes a 2.2-fold decrease in Ni-induced lipid peroxidation in shoot tissue, measured as the presence of thiobarbituric acid reactive species (TBARS; Fig. 2). SA treatment, which caused significant increases in SA accumulation in shoots (Fig. 3), was also observed to induce a linear increase in SAT activity in Arabidopsis (Fig. 3A), along with increases in its product O-acetyl-l-Ser (OAS) and the downstream metabolite GSH (Fig. 3, B and C). The enzyme responsible for maintaining a reduced pool of GSH, GR is also activated after SA exposure (Fig. 3D). Furthermore, these metabolic changes are also observed in the SA-signaling mutant npr1-1 (Fig. 3), suggesting that SAT activity is regulated by SA in an NPR1-independent manner. Increases in GSH, driven by overexpression of SAT, have previously been shown by the authors to decease the level of both Ni induced reactive oxygen species (ROS) and lipid peroxidation in Arabidopsis (Freeman et al., 2004), establishing the link between elevated GSH and reduced Ni-induced ROS. Qualitative measurement of the different SAT mRNAs by reverse transcription (RT)-PCR reveals that SA exposure does not cause changes in steady-state SAT mRNA (Fig. 4A), and immunoblotting using antiserum that cross-reacts with all three SAT isoforms shows no major changes in the accumulation of SAT (Fig. 4B) in either wild-type or npr1-1. Based on this evidence, SA appears to regulate SAT activity posttranslationally via an NPR1-independent pathway. Such regulation is consistent with the observation that SA pretreatment induces enhanced accumulation of GSH and protection against Ni toxicity, since GSH is known to protect against Ni-induced lipid peroxidation in Arabidopsis and Thlaspi hyperaccumulators (Freeman et al., 2004).
Figure 2.
SA protection against Ni-induced lipid peroxidation in Arabidopsis. Arabidopsis (Col) were grown on solidified one-half Murashige and Skoog + B5 plates for 7 d then transferred to medium containing 0 or 50 μm SA for 14 d prior to transfer onto medium lacking SA but containing 100 μm Ni(NO3)2. After a further 8 d, shoot tissue was harvested and analyzed for TBARS. Data represents mean (n = 9) ± sd. Different lowercase letters represent a significant difference between means (Student's t test P > 0.01).
Figure 3.
Effects of SA treatment on GSH metabolism. Arabidopsis was germinated and grown on solidified one-half Murashige and Skoog + B5 vitamin medium containing 0, 50, or 100 μm SA. After 14 d, shoot tissue was harvested and analyzed for SA and SAT activity (A), OAS (B), total GSH (C), and GR activity (D) in both wild-type Arabidopsis (Ws; circles) and npr1-1 (triangles). Control levels of SAT activity, OAS, total GSH, and GR in Arabidopsis were 0.05 nmol OAS min−1 mg−1 total protein, 0.015 nmol g−1 fresh weight, 260.9 nmol g−1 fresh weight, and 11.7 nmol GSH mg−1 total protein min−1, respectively, and 0.16 nmol OAS min−1 mg−1 total protein, 0.013 nmol g−1 fresh weight, and 271.4 nmol g−1 fresh weight, respectively, in npr1-1. Data for control values represent an average of three to six independent replicate analyses. GR activity and corresponding SA levels were measured on equivalent replicate samples, and data represent the average (n = 3) ± sd.
Figure 4.
Transcriptional and translational regulation of SAT by SA and Ni2+ in Arabidopsis. Both wild-type Arabidopsis and npr1-1 were grown on solidified one-half Murashige and Skoog + B5 vitamin medium for 14 d prior to transfer onto medium containing various concentrations of SA or Ni2+. After a further 14 d, shoot tissue was harvested and analyzed by RT-PCR for steady-state levels of SAT mRNA (A) and by immunoblotting for SAT protein.
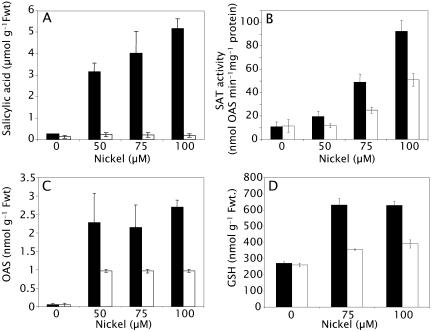
To develop further evidence for a role of SA in regulating GSH-mediated Ni resistance via activation of SAT, we observed that the SA-signaling mutant npr1-1 hyperaccumulates SA on exposure to Ni (Fig. 5A). This accumulation of SA in npr1-1 is associated with enhanced increases in SAT activity (Fig. 5B), OAS, and GSH (Fig. 5, C and D), in a similar manner to that observed in wild-type Arabidopsis after exposure to exogenous SA (Fig. 3). The enhanced SAT activity observed in npr1-1 after Ni exposure is not related to increased SAT mRNA (Fig. 4A) or SAT protein (Fig. 4B), suggesting a posttranslational regulatory mechanism, as observed for SA induced SAT activity (Figs. 3 and 4). These metabolic changes in npr1-1 are also associated with increased Ni resistance (Figs. 6 and 7A) similar to that observed in SA-pretreated plants (Fig. 2). Mutation of NPR1 leads to a 2-fold increase in Ni resistance, quantified as an increase in the I50 for inhibition of root growth from 75 μm to 150 μm Ni (Fig. 7B). Such Ni resistance in npr1-1 is completely abolished when plants are grown in the presence of the GSH biosynthetic inhibitor buthionine sulfoximine (Meister, 1988; Fig. 7D), strongly supporting a role for GSH in the enhanced Ni resistance of npr1-1. Though significant, this increase in Ni resistance in npr1-1 is still lower than that observed in the Ni hyperaccumulator T. goesingense (Fig. 7, A and B). Interestingly, Arabidopsis wild-type, npr1-1, and T. goesingense accumulate equal amounts of Ni over the range of Ni concentrations in the medium at which the plants are viable (Fig. 7C), confirming that Ni tolerance rather than Ni uptake rates are what distinguish the hyperaccumulator T. goesingense from the nonaccumulator (Krämer et al., 1997), at least in in vitro culture.
Figure 5.
Regulation of SA and GSH metabolism by Ni2+ in wild-type Arabidopsis and the SA nonresponsive npr1-1 mutant. Both wild-type Arabidopsis and npr1-1 were grown on solidified one-half Murashige and Skoog + B5 vitamin medium for 7 d prior to transfer onto medium containing 0, 50, 75, or 100 μm Ni(NO3)2. After 14 d, shoot tissue was harvested and analyzed for SA (A), SAT activity (B), OAS (C), and total GSH (D). Bars represent wild type (white) and npr1-1 (black). Data represent the mean (n = 10) ± sd.
Figure 6.
Ni resistance of wild-type Arabidopsis and npr1-1 mutant. Both wild-type Arabidopsis and npr1-1 were germinated and grown on solidified one-half Murashige and Skoog + B5 vitamin medium without (A) and with (B) 100 μm Ni(NO3)2 for 30 d.
Figure 7.
Ni resistance and accumulation of wild-type Arabidopsis, npr1-1, and T. goesingense (Tg). A, Plants were germinated and grown for 30 d on solidified one-half Murashige and Skoog + B5 vitamin medium containing various concentrations of Ni(NO3)2. B, Root lengths plotted as a percentage of the root length after growth in the absence of Ni for T. goesingense (circles), Arabidopsis npr1-1 (squares), and Arabidopsis wild type (triangles). Data represent means (n = 20) ± sd. C, Nickel concentrations in shoot tissue of T. goesingense (circles), Arabidopsis npr1-1 (squares), and Arabidopsis wild type (triangles). Data represent means (n = 9) ± sd. D, Wild-type Arabidopsis (white bars) and npr1-1 (black bars) were germinated and grown on solidified one-half Murashige and Skoog + B5 vitamin medium containing various combinations of 100 μm Ni(NO3)2 and 100 μm buthionine sulfoximine. After 30 d, root lengths were measured and data reported as mean (n = 30) ± sd. Lowercase letters (a, b, and c) represent significantly different means using the mixed procedure function in SAS (P < 0.01).
DISCUSSION
Elevated concentrations of free SA are found to be a strong predictor of Ni hyperaccumulation across six different species of Thlaspi hyperaccumulator and nonaccumulators (Tables I and II). Furthermore, the SA upstream metabolites Phe and cinnamic acid, from the Phe ammonia lyase SA biosynthetic pathway, are also elevated in the hyperaccumulator, along with the downstream metabolites salicyloyl-Glc and catechol (Table I). Such perturbations suggest SA metabolism in the Thlaspi hyperaccumulators is permanently activated. SA is known to be involved in plant PRs, including signaling both the hypersensitive response (HR) and systemic acquired resistance (for review, see Shah, 2003). Furthermore, SA has been implicated in plant responses to heavy metals, including Pb and Hg in rice (Mishra and Choudhuri, 1999), and Cd in barley and maize seedlings (Pál et al., 2002; Metwally et al., 2003). However, the mechanism of SA-mediated metal resistance was not elucidated. Cadmium exposure has also been shown to induce accumulation of SA (Metwally et al., 2003), and exposure to Al, Cu, and Cd elicits expression of a pathogenesis related (PR2) protein in wheat (Triticum aestivum) roots (Cruz-Ortega and Ownby, 1993). Such observations suggest that plant responses to both metal toxicity and pathogens share certain mechanistic commonalities, possibly signaled by the formation of ROS (Overmyer et al., 2003). Our observation that preexposure of Arabidopsis to SA reduces Ni toxicity and increases SAT activity and accumulation of GSH supports a connection between SA and GSH-mediated Ni resistance. Furthermore, SA appears to act by regulating SAT activity posttranslationally, since direct assays of SAT, measured as production of OAS after addition of the substrates Ser and acetyl-CoA to a desalted extract, show increased activity without increases in either SAT mRNA or protein. Such increased SAT-specific activity observed in vitro could be due to either altered phosphorylation status or alteration of the stability of the regulatory complex between SAT and OAS (thiol) lyase, both known to alter SAT activity in plants (Bogdanova and Hell, 1997; Yoo and Harmon, 1997). Reduction in the Cys sensitivity of one or more of the SAT isoforms (Inoue et al., 1999), possibly mediated by phosphorylation, could also contribute to the increased in vivo SAT activity suggested by the elevation of OAS after SA exposure. Answers to these intriguing questions await further study.
Increases in SAT activity and GSH were also observed in the SA-signaling mutant npr1-1, demonstrating that this regulation of SAT and GSH is independent of the NPR1 signaling pathway. The signaling mutant npr1-1 lacks the ability to negatively regulate SA levels and SA hyperaccumulates in this mutant after challenge with a pathogen (Delaney et al., 1995) or after Ni exposure (Fig. 5A), demonstrating that both pathogens and Ni activate SA accumulation, possibly signed via an oxidative burst. SA hyperaccumulation in npr1-1 was also found to be associated with increased SAT activity, GSH accumulation, and increased Ni resistance in this mutant. Based on such evidence, we propose that constitutively elevated SA in the hyperaccumulators acts to posttranslationally up-regulate SAT activity, causing constitutively elevated GSH and Ni tolerance, as previously observed in these hyperaccumulator species (Freeman et al., 2004).
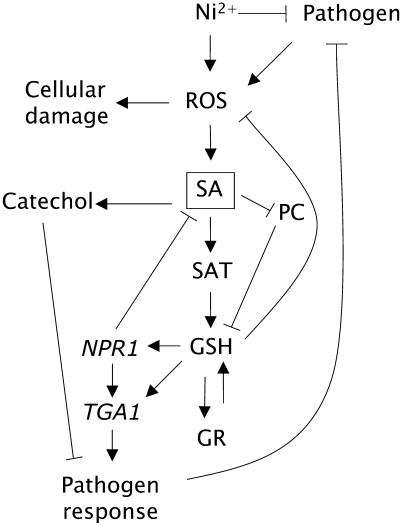
Based on such observations, we propose the following model for the role of SA in Thlaspi Ni hyperaccumulators (Fig. 8). SA activates SAT posttranslationally causing accumulation of GSH and activation of GR (Knörzer et al., 1996) to maintain an enhanced pool of reduced GSH (Freeman et al., 2004). Increased GSH pools allow Ni-hyperaccumulating Thlaspi to resist Ni-induced oxidative stress (Freeman et al., 2004). Furthermore, SA potentially blocks phytochelatin synthase activity (Pál et al., 2002), inhibiting phytochelatin biosynthesis in response to Ni and conserving GSH to act as an antioxidant. Such inhibition is consistent with the lack of accumulation of phytochelatin-Ni complexes in T. goesingense (Freeman et al., 2004). The recent paradoxical observation that overexpression of phytochelatin synthase activity confers Cd sensitivity in Arabidopsis supports this view that depletion of GSH pools for phytochelatin synthesis can be detrimental to metal tolerance (Lee et al., 2003). Considering that SA normally signals HR and systemic acquired resistance in the nonaccumulator Arabidopsis (for review, see Dempsey et al., 1999), it is curious how Thlaspi hyperaccumulators are able to maintain constitutively high SA levels without initiating a PR. We propose that in the hyperaccumulator, the elevated levels of catecol, a potential breakdown product of SA, inhibits the initiation of a PR, as proposed previously in Arabidopsis (Van Wees and Glazebrook, 2003). However, it is also possible that mutations in other components of the SA-signaling cascade may exist in Thlaspi hyperaccumulators. This remains to be determined. The observation that in the absence of Ni in the growth medium T. goesingense is more susceptible to powdery mildew and does not increase SA synthesis or show any visible signs of a PR supports the conclusion that SA-mediated PRs in T. goesingense are suppressed. A similar increase in sensitivity to powdery mildew (Erysiphe orontii) occurs in the Arabidopsis mutant npr1-1 (Reuber et al., 1998), and npr1-1 also suppresses the increased resistance to powdery mildew (Erysiphe cichoracearum) of edr1 (Frye et al., 2001). Normally, NPR1 acts to negatively regulate SA and signal SA-dependant gene expression via interaction with Leu zipper transcription factors, including TGA1. SA-dependent activation of NPR1, and its interaction with TGA1, has recently been shown to require reduction of critical disulfides in both proteins (Despres et al., 2003; Mou et al., 2003). Our observation of the SA-dependant activation of SAT, and the concomitant accumulation of GSH, provides one possible mechanism for such reduction via increased GSH levels. In npr1-1, SA-dependent signaling of GSH accumulation remains functional, allowing enhanced resistance to Ni. Furthermore, the inability of npr1-1 to sense SA potentially suppresses its ability to initiate HR in response to the oxidative burst associated with Ni exposure, possibly further enhancing the Ni resistance of npr1-1. The fact that accumulation of Ni in T. goesingense enhances resistance to E. cruciferarum (Fig. 1) suggests that Ni hyperaccumulation may be compensating for the loss of normal PRs in these plants. Such a model has significant implications for both the mechanism of Ni tolerance in Thlaspi hyperaccumulators and the selective pressures driving their evolution.
Figure 8.
Model of the role of SA in response to biotic and abiotic Ni stress in Thlaspi hyperaccumulators. PC, Phytochelatins; NPR1, nonexpressor of PR genes; TGA1, basic domain/Leu zipper transcription factor.
MATERIALS AND METHODS
Plant Material
Seeds of the Ni hyperaccumulator Thlaspi goesingense (Hálácsy) were collected from an ultramafic site in Redschlag, Austria (Krämer et al., 1997). Shoot tissue samples from the Redschlag population of T. goesingense were collected, rapidly frozen on site, shipped to the United States in dry ice, and frozen at −80°C before extraction and HPLC analysis of SA levels. Seeds of the nonaccumulator Thlaspi arvense were collected from a calcareous soil at Col de Gleize, France (44°37′164″N, 6°03′959″E). All other accessions were as described by Peer et al. (2003) except seeds of Arabidopsis (Arabidopsis thaliana; Ws and Col-0), which were purchased from Lehle seeds (Round Rock, TX). Arabidopsis and various Thlaspi species were grown in a growth room (24°C/20°C, 10 h/14 h light/dark, 120 μmol m−2 s−1 photosynthetic photon flux) in artificial soil mix (Metro mix; Scotts, Marysville, OH). Arabidopsis was grown for 5 weeks and T. goesingense for 6 weeks, and plants compared were of equal size and leaf number. Shoot tissue was harvested at the rosette stage 5 h after the onset of the light period and immediately frozen in liquid nitrogen. Plants propagated on plates were grown on one-half strength Murashige and Skoog medium + B5 vitamins containing 0.8% agar on light racks in an environmentally controlled room (24°C/20°C, 14 h/10 h light/dark, 120 μmol m−2 s−1 photosynthetic photon flux).
Metabolite Quantification
Liquid N2 frozen tissue stored at −80°C was extracted in ice-cold methanol at 4°C for 24 h, then phase extracted in ice-cold water/chloroform at 4°C for 12 h (Rhodes et al., 1986), with the addition of a norvaline internal standard. Extracts were derivatized and analyzed for all amino acids including OAS and Phe using AccQ Tag amino acid analysis following manufacturer's instructions (Waters, Milford, MA). SA, salicyloyl-Glc, catechol, and cinnamic acid were quantified by HPLC at 25°C using a Nova-Pak C-18 column with a flow rate of 1 mL min−1 over 22 min using a methanol gradient (solvent A, water and 1% formate; and solvent B, 100% methanol and 1% formate) of 10% to 40% B (10 min), 40% to 50% B (5 min), 50% to 100% B (2.5 min), 100% to 40% B (2.5 min), 40% to 10% B (1 min), and 10% B (1 min). Phenolic compounds were detected using both fluorescence (Ex254 and Em395) and A280. Analyses were performed using Waters Alliance HPLC system equipped with Millenium software, 2695 Separations Module, 2475 fluorescence detector, and 2996 Photodiode array detector. Standard curves were established using amino acid standard H (Pierce Chemical, Rockford, IL; catalog no. NCI0180), OAS (Sigma-Aldrich, St. Louis; catalog no. A–6262), SA (Sigma-Aldrich; catalog no. S–6271), cinnamic acid (Sigma-Aldrich; catalog no. 96340), and catechol (Sigma-Aldrich; catalog no. C9510). Identity of SA, salicyloyl-Glc, catechol, and cinnamic acid from plant samples were confirmed by liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS). Extracts from T. goesingense and other Thlaspi hyperaccumulators were fractionated as described above for phenolics and 30 s fractions collected. Fractions corresponding to the correct retention time and with the correct UV absorption spectrum were taken to dryness, resuspended in 50% methanol, and identified using accurate mass LC-MS/MS by identification of the correct parent ion with a Waters system equipped with MassLynx 4.0 software, AllianceHT Separations Module, 2996 Photodiode array detector, and Micromass Q-Tof micro mass spectrometer. Identity of SA and salicyloyl-Glc was further confirmed after fragmentation of the appropriate parent ions. Identification of OAS was confirmed using gas chromatography-mass spectrometry after methanol/chloroform/water extraction, standard amino acid fractionation (Rhodes et al., 1986), and derivatization with N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide in pyridine. Thiols were derivatized with monobromobimane and quantified using HPLC as described (Tsakraklides et al., 2002).
Enzyme Assays
Freshly frozen Arabidopsis leaf tissue was immediately assayed for SAT activity in Sephadex G-25 fine desalted plant extracts after homogenization in 100 mm KH2PO4/K2HPO4, pH 7.6, by quantification of OAS produced after addition of Ser and acetyl-CoA, with a final reaction mixture of 100 μL containing 1 mm l-Ser, 0.1 mm acetyl-CoA, 1.25 mm Na2EDTA, and 0.1 mm norvaline (internal standard) in 100 mm KH2PO4/K2HPO4, pH 7.6, at 25°C (Błaszcyk et al., 2002). Enzyme assays were stopped during the linear phase of the reaction at 20 min by the addition of ice-cold methanol/chloroform (2:1, v/v) and stored overnight at −80°C. OAS in the aqueous phase was derivatized and quantified using AccQ Tag following the manufacturer's instructions (Waters). GR was assayed in desalted total plant extracts in 100 mm Na2HPO4, 1 mm EDTA, pH 7.5, with HCl, using dithionitrobenzoate (Smith et al., 1988).
Immunoblot Analysis
SDS-PAGE was performed as described previously (Laemmli, 1970). Crude protein extracts from Arabidopsis were obtained by grinding shoot tissue samples in liquid nitrogen and mixing the frozen powdered plant material in a 2:1 ratio (w/v) with SDS sample buffer. The mixture was boiled for 10 min and centrifuged at 16,000g, and the supernatant was assayed for total protein using bicinchoninic acid (Pierce Chemical), and equal amounts of protein (30 μg) loaded onto an SDS-PAGE gel. For immunoblotting, proteins were transferred from the SDS-PAGE gel onto an Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) using electrophoretic semidry blotting. Ser acetyltransferase was visualized on the membrane using polyclonal primary antibodies raised against Arabidopsis SAT-m in rabbits and a secondary anti-IgG antibody raised in goat and conjugated to alkaline phosphatase. Blots were developed by the addition of nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate.
RT-PCR-Based Assessment of SAT Transcript Levels
For RT-PCR analysis, 5 μg of total RNA was isolated from Arabidopsis (Col-0) and npr1-1 shoot tissue after SA or Ni treatments and used as templates for RT using Superscript II (Invitrogen, Carlsbad, CA). Arabidopsis SAT-m, SAT-p, SAT-c, and β-tubulin were amplified from the RT reaction by PCR (94°C 5 min [94°C 30 s, 60°C 1 min, 72°C 2 min] × 30 cycles and 72°C 5 min) using Taq DNA polymerase (Promega, Madison, WI) and the appropriate pair of gene-specific primers (SAT-m, forward, 5′-GTCACAAGTCGCCGCCACTTCACA-3′ and reverse, 5′-AATTACATAATCCGACCACTCGG-3′; SAT-p, forward, 5′-TGCATCCACACATGCCGAACCGGT-3′ and reverse, 5′-AATTACATAATCCGACCACTCGG-3′; SAT-c, forward, 5′-GCCGGAGAACTCCGACATCAATCT-3′ and reverse, 5′-TATGATGTAGTCTGACCATTCCGA-3′; and β-tubulin forward, 5′-CGTGGATCACAGCAATCAAGAGCC-3′ and reverse, 5′-CCTCCTGCACTTCCACTTGGTCTTC-3′).
Quantification of Shoot Nickel Concentrations
After appropriate growth periods, shoot tissue was carefully harvested for inductively coupled plasma MS analysis of metal levels. Metal analysis was performed on dried shoot tissue as described previously (Lahner et al., 2003).
Quantification of Total Lipid Peroxidation
Peroxidized lipids were assayed as the presence of TBARS. Shoot tissue was harvested and assayed for TBARS as described previously (Murphy et al., 1999). During the period of metal exposure, all plants remained viable and continued growing.
Statistical Methods
All statistical analyses were performed using SAS software version 8e (SAS Institute, Cary, NC).
Acknowledgments
D.E.S. conceived the experiment with contributions from J.L.F., J.L.F. was primarily responsible for carrying it out, with D.G. and A.H. contributing to metabolite analyses, D.K. to RT-PCR and immunoblotting; D.E.S. and J.L.F. cowrote the paper. We also acknowledge the Purdue Ionomics Center and Brett Lahner for inductively coupled plasma analyses, Purdue Discovery Park for LC-MS/MS, Ralph Nicholson and Uwe Braun for work with the pathogen, Walter Wenzel for field collection of T. goesingense tissue, and Thomas Sors for assistance with statistical analyses.
This work was supported by the U.S. National Science Foundation (grant nos. 0196310–IBN and 0129747–IBN to D.E.S.) and by the Indiana 21st Century Research and Technology Fund.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055293.
References
- Adam L, Ellwood S, Wilson I, Saenz G, Xiao S, Oliver RP, Turner JG, Somerville S (1999) Comparison of Erysiphe cichoracearum and E. cruciferarum and a survey of 360 Arabidopsis thaliana accessions for resistance to these two powdery mildew pathogens. Mol Plant Microbe Interact 12: 1031–1043 [DOI] [PubMed] [Google Scholar]
- Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161: 319–328 [DOI] [PubMed] [Google Scholar]
- Assunção AGL, Martins PD, De Folter S, Vooijs R, Schat H, Aarts MGM (2001) Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ 24: 217–226 [Google Scholar]
- Becher M, Talke IN, Krall L, Krämer U (2004) Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J 37: 251–268 [DOI] [PubMed] [Google Scholar]
- Bezrukova MV, Sakhabutdinova R, Fatkhutdinova RA, Kyldiarova I, Shakirova F (2001) The role of hormonal changes in protective action of salicylic acid on growth of wheat seedlings under water deficit. Agrochemiya (Russ) 2: 51–54 [Google Scholar]
- Błaszcyk A, Sirko L, Hawkesford MJ, Sirko A (2002) Biochemical analysis of transgenic tobacco lines producing bacterial serine acetyltransferase. Plant Sci 162: 589–597 [Google Scholar]
- Bogdanova N, Hell R (1997) Cysteine synthesis in plants: protein-protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J 11: 251–262 [DOI] [PubMed] [Google Scholar]
- Boyd RS, Shaw JJ, Martens SN (1994) Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens. Am J Bot 81: 294–300 [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Cruz-Ortega R, Ownby JD (1993) A protein similar to PR (pathogenesis-related) proteins is elicited by metal toxicity in wheat roots. Physiol Plant 89: 211–219 [Google Scholar]
- Dat J, Foyer C, Scott I (1998) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118: 1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92: 6602–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18: 547–575 [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR (2003) The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell 15: 2181–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279–290 [PMC free article] [PubMed] [Google Scholar]
- Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7: 547–552 [DOI] [PubMed] [Google Scholar]
- Dräger DB, Desbrosses-Fonrouge AG, Krach C, Chardonnens AN, Meyer RC, Saumitou-Laprade P, Kramer U (2004) Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J 39: 425–439 [DOI] [PubMed] [Google Scholar]
- Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16: 2176–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Tang D, Innes RW (2001) Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA 98: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Salt DE (2001) Fortified foods and phytoremediation: two sides of the same coin. Plant Physiol 125: 164–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Noji M, Saito K (1999) Determination of the sites required for the alloseric inhibition of serine acetyltransferase by L-cysteine in plants. Eur J Biochem 266: 220–227 [DOI] [PubMed] [Google Scholar]
- Janda T, Szalai G, Tari I, Paldi E (1999) Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208: 175–180 [Google Scholar]
- Kachroo P, Yoshioka K, Shah J, Dooner HK, Klessig DF (2000) Resistance to turnip crinkle virus in Arabidopsis is regulated by two host genes and is salicylic acid dependent but NPR1, ethylene, and jasmonate independent. Plant Cell 12: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Gustin JL, Lahner B, Persans MW, Baek D, Yun DJ, Salt DE (2004) The plant CDF family member TgMTP1 from the Ni/Zn hyperaccumulator Thlaspi goesingense acts to enhance efflux of Zn at the plasma membrane when expressed in Saccharomyces cerevisiae. Plant J 39: 237–251 [DOI] [PubMed] [Google Scholar]
- Kinkema M, Fan W, Dong X (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12: 2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knörzer OC, Durner J, Boger P (1996) Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol Plant 97: 388–396 [Google Scholar]
- Knörzer OC, Lederer B, Durner J, Böger P (1999) Antioxidant defense activation in soybean cells. Physiol Plant 107: 294–302 [Google Scholar]
- Krämer U, Pickering IJ, Prince RC, Raskin I, Salt DE (2000) Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol 122: 1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Smith RD, Wenzel W, Raskin I, Salt DE (1997) The role of nickel transport and tolerance in nickel hyperaccumulation by Thlaspi goesingense Hálácsy. Plant Physiol 115: 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Lombi E, Zhao F-J, Wieshammer G, McGrath SP (2001) Cellular compartmentalization of nickel in the hyperaccumulator Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot 52: 2291–2300 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680–685 [DOI] [PubMed] [Google Scholar]
- Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE, Guerinot ML, Harper JF, Ward JM, McIntyre L, et al (2003) Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 21: 1215–1221 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight M (2002) Protection against heat stress induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131: 656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León J, Lawton M, Raskin I (1995) Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol 108: 1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409: 579. [DOI] [PubMed] [Google Scholar]
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205–17208 [PubMed] [Google Scholar]
- Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132: 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Choudhuri MA (1999) Effect of salicylic acid on heavy metal-induced membrane deterioration in rice. Biol Plant 42: 409–415 [Google Scholar]
- Mou Z, Fan W, Dong X (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113: 935–944 [DOI] [PubMed] [Google Scholar]
- Murphy AS, Eisinger WR, Shaff JE, Kochian LV, Taiz L (1999) Early copper-induced leakage of K+ from Arabidopsis seedlings is mediated by ion channels and coupled to citrate efflux. Plant Physiol 121: 1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Pál M, Szalai G, Horvath E, Janda T, Paldi E (2002) Effect of salicylic acid during heavy metal stress. Proc 7th Hungarian Cong Plant Phys 46: 119–120 [Google Scholar]
- Peer WA, Mamoudian M, Lahner B, Reeves RD, Murphy AS, Salt DE (2003) Identifying model metal hyperaccumulating plants: germplasm analysis of 20 Brassicaceae accessions from a wide geographical area. New Phytol 159: 421–430 [DOI] [PubMed] [Google Scholar]
- Persans MW, Nieman K, Salt DE (2001) Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc Natl Acad Sci USA 98: 9995–10000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, Van Loon LCV (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7: 1–9 [DOI] [PubMed] [Google Scholar]
- Raskin I, Skubatz H, Tang W, Meeuse BJD (1990) Salicylic acid levels in thermogenic and non-thermogenic plants. Ann Bot (Lond) 66: 369–373 [Google Scholar]
- Reeves RD, Baker AJM (2000) Metal-accumulating plants. In I Raskin, BD Ensley, eds, Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment. John Wiley & Sons, New York, 193–229
- Reeves RD, Brooks RR (1983) European species of Thlaspi L. (Cruciferae) as indicators of nickel and zinc. J Geochem Explor 18: 275–283 [Google Scholar]
- Reeves RD, Schwartz C, Morel JL, Edmondson J (2001) Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France. Int J Phytoremediat 3: 145–172 [Google Scholar]
- Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W, Ausubel FM (1998) Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J 16: 473–485 [DOI] [PubMed] [Google Scholar]
- Rhodes D, Deal L, Haworth P, Jamieson GC, Reuter CC, Ericson MC (1986) Amino acid metabolism of Lemna minor L.: responses to methionine sulfoximine. Plant Physiol 82: 1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, et al (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 9: 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Kato N, Krämer U, Smith RD, Raskin I (1999) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and non-accumulator species of Thlaspi. In N Terry, GS Bañuelos, eds, Phytoremediation of Contaminated Soil and Water, Chapter 10. CRC Press, Boca Raton, FL, pp 191–202
- Shah J (2003) The salicylic acid loop in plant defence. Curr Opin Plant Biol 6: 365–371 [DOI] [PubMed] [Google Scholar]
- Sharma Y, Leon J, Raskin I, Davis K (1996) Ozone-induced responses in Arabidopsis thaliana: the role of salycylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA 93: 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5′-Dithiobis(2-nitrobenzoic acid). Anal Biochem 175: 408–413 [DOI] [PubMed] [Google Scholar]
- Srivastava M, Dwivedi U (1998) Salicylic acid modulates glutathione metabolism in pea seedlings. J Plant Physiol 153: 409–414 [Google Scholar]
- Takahashi H, Miller J, Nozaki Y, Takeda M, Shah J, Hase S, Ikegami M, Ehara Y, Dinesh-Kumar SP (2002) RCY1, an Arabidopsis thaliana RPP8/HRT family resistance gene, conferring resistance to cucumber mosaic virus requires salicylic acid, ethylene and a novel signal transduction mechanism. Plant J 32: 655–667 [DOI] [PubMed] [Google Scholar]
- Tari I, Csiszar J, Szalai G, Horvath F, Pecsvaradi A, Kiss G, Szepesi A, Szabo M, Erdei L (2002) Acclimation of tomato plants to salinity stress after a salicylic acid pre-treatment. Proc 7th Hungarian Congress Plant Phys 46: 55–56 [Google Scholar]
- Tsakraklides G, Martin M, Chalam R, Tarczynski M, Schmidt M, Leustek T (2002) Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J 32: 879–889 [DOI] [PubMed] [Google Scholar]
- Vacchina V, Mari S, Czernic P, Marquès L, Pianelli K, Schaumlöffel D, Lebrun M, Łobiński R (2003) Speciation of nickel in a hyperaccumulating plant by high-performance liquid chromatography-inductively coupled plasma mass spectroscopy and electrospray MS/MS assisted by cloning using yeast complementation. Anal Chem 75: 2740–2745 [DOI] [PubMed] [Google Scholar]
- Van Wees SCM, Glazebrook J (2003) Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J 33: 733–742 [DOI] [PubMed] [Google Scholar]
- Weber M, Harada E, Vess C, Roepenack-Lahaye EV, Clemens S (2004) Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J 37: 269–281 [DOI] [PubMed] [Google Scholar]
- Wenzel WW, Jockwer F (1999) Accumulation of heavy metals in plants grown on mineralized soils of the Austrian Alps. Environ Pollut 104: 145–155 [Google Scholar]
- Yoo BC, Harmon AC (1997) Regulation of recombinant soybean serine acetyltransferase by CDPK. Plant Physiol (Suppl) 114: 267 [Google Scholar]