Summary
The causality between atherosclerosis and dementia remains unclear. This study aimed to explore the causal effect of atherosclerosis related indicators on dementia risk based on two-sample Mendelian randomization (MR) using summary statistics of genome-wide association studies (GWASs). The inverse variance weighted (IVW) method was performed as the main analysis, supplemented by different sensitivity analyses. Suggestive evidence indicated that peripheral arterial disease (PAD) (odds ratio (OR): 0.864, 95% confidence interval (CI): 0.797–0.937), coronary atherosclerosis (CoAS) (OR: 0.927, 95% CI: 0.860–0.998) and atherosclerosis, excluding cerebral, coronary, and PAD (ATHSCLE) (OR: 0.812, 95% CI: 0.725–0.909) were inversely associated with the risk of AD. The sensitivity analysis confirmed a suggestive reverse effect of ATHSCLE on the risk of frontotemporal dementia (FTD) (OR, 0.812, 95% CI, 0.725–0.909). Findings provide suggestive evidence that PAD, CoAS, and ATHSCLE might be associated with the risk of AD or FTD, which requires further exploration in larger samples.
Subject areas: Cardiovascular medicine, Public health, Human genetics, Clinical neuroscience
Graphical abstract

Highlights
-
•
No valid causal effect of subclinical atherosclerosis on dementia was obtained
-
•
Pleiotropic SNPs and small sample size may be key to the robustness of the results
-
•
GWASs with larger sample size are needed for further causal inference
Cardiovascular medicine; Public health; Human genetics; Clinical neuroscience
Introduction
Dementia, mainly including Alzheimer’s disease (AD) (accounting for 50–70%), dementia with Lewy bodies (DLB) (accounting for 10%–15%), frontotemporal dementia (FTD) (accounting for 5%–10%), and vascular dementia (VaD) (accounting for 15%–20%), is affecting about 50 million people worldwide and is expected to rise to 152 million by 2050.1,2,3 The main clinical manifestation of dementia and its subtypes is a progressive impairment in cognitive function, language, and behavior, which poses a serious financial and healthcare burden on patients, families, medical institutions, and society.4,5 Given the lack of effective drugs for dementia and the fact that preclinical changes in the brain may occur long before dementia develops, early identification, prevention, and intervention of potential modifiable risk factors for dementia is of great significance.
Arterial stiffness and atherosclerosis, two well-documented modifiable vascular risk factors, contribute to the initiation and development of age-related cognitive impairment and dementia.6,7,8,9 Arterial stiffness, which affects the natural cushioning function of the arterial system (including cerebral vessels) and eventually brain function, has emerged as an independent predictor of vascular aging and has been suggested to be related to atherosclerosis.10 Pulse wave velocity (PWV), especially carotid-femoral PWV (cfPWV), is the reference standard for measuring arterial stiffness, while arterial stiffness index (ASI) is a marker that can be measured noninvasively using pulse waveforms.11,12 The atherosclerotic process, characterized by atherosclerotic plaque formation, stenosis, and blockage of arteries,13 has a long preclinical phase called subclinical atherosclerosis, and noninvasive methods can be used for early detection and prevention.14,15 Some typical examples include associations between noninvasive indicators or markers of subclinical atherosclerosis or arterial stiffness (e.g., carotid plaque burden, peripheral arterial disease (measured as ankle-brachial index (ABI)), and PWV), and cognitive function.6,16,17,18,19,20 In addition, a clinical study supports the relationship between carotid atherosclerosis (CAS) and VaD, as well as AD.21 However, owing to the potential biases from residual confounding and reverse causality, inconsistent correlation conclusions were found in the published observational studies mentioned previously. Although some evidence suggests that atherosclerosis and dementia may share a common pathogenetic pathway,22,23,24 the genetic causality of this association remains to be further explored.
A Mendelian randomization approach exploits the natural random assignment of genetic variants related to hypothesized risk factors to improve causal inference when using observational data, which is not susceptible to resident confounders and reverse causality.25 In this study, we leveraged summary-level data of GWAS of European ancestry to identify the causal effect of arterial stiffness and atherosclerotic indicators on the risk of dementia and its subtypes. The GWAS summary data in this study are detailed in Table 1 and an overview of the study design is shown in Figure 1.
Table 1.
Data sources used for the two-sample Mendelian randomization analysis
| Phenotype | Phenocode/GWAS ID | Sample size (cases/controls) | Consortium or cohort study (Link URL) | |
|---|---|---|---|---|
| Exposures | CPAmax/CPSmax | 81W59K3RUJ-4/81W5GAJXK9-2 | 1,277 individuals | Cohort study, https://www.health-atlas.de/ |
| baPWV/bfPWV | 82VNGAUQKT-0 | 3,643-6,734 individuals | Cohort study, https://www.health-atlas.de/ | |
| PAD | 1) ukb-d-I9_PAD (original) 2) I9_PAD (PAD_FinnGen) (sensitivity) |
1) 1,230/359,964 2) 9,021/244,907 |
NA, IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) R6, FinnGen consortium (https://www.finngen.fi/en) |
|
| pwASI | ukb-b-11971 | 151,053 individuals | MRC-IEU, IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) | |
| CoAS | I9_CORATHER (FinnGen) | 42,421/285,621 | R8, FinnGen consortium (https://www.finngen.fi/en) | |
| CeAS | I9_ CEREBATHER (FinnGen) | 282/342,217 | R8, FinnGen consortium (https://www.finngen.fi/en) | |
| ATHSCLE | I9_ ATHSCLE (FinnGen) | 13,434/317,899 | R8, FinnGen consortium (https://www.finngen.fi/en) | |
| CAD | ebi-a-GCST005195 | 122,733/547,261 | IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) | |
| Outcomes | AD | 1) ieu-b-2 (original) 2) F5_ALZHDEMENT (AD_FinnGen) (sensitivity) |
1) 21,982/41,944 2) 4,406/325,306 |
1) IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) 2) R8, FinnGen consortium (https://www.finngen.fi/en) |
| DLB | ebi-a-GCST90001390 | 2,591/4,027 | NA, IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) | |
| FTD | 1) ieu-b-43 (original) 2) FTD_meta (sensitivity) 3) FTD_FinnGen (sensitivity) |
1) 515/2,509 2) 2,154/4,308 3) 99/328,323 |
1) NA, IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) 2) https://ifgcsite.wordpress.com/data-access/(FTD meta)26 3) R8, FinnGen consortium (https://www.finngen.fi/en) |
|
| VaD | F5_VASCDEM | 2,048/328,982 | R8, FinnGen consortium (https://www.finngen.fi/en) | |
| Any dementia | KRA_PSY_DEMENTIA_EXMORE | 12,042/254,976 | R8, FinnGen consortium (https://www.finngen.fi/en) |
CPAmax, area of the largest carotid plaque burden detected; CPSmax, maximal degree of stenosis; baPWV, brachial-ankle pulse wave velocity; bfPWV, brachial-femoral; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, atherosclerosis, excluding cerebral, coronary and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; GWAS, genome-wide association study
Figure 1.
Overview of the MR study design
The dashed lines represent pathways that violate the MR assumptions. SNP, single nucleotide polymorphism; CPAmax, area of the largest carotid plaque detected; CPSmax, maximal degree of stenosis; PWV: pulse wave velocity; baPWV, brachial-ankle PWV; bfPWV, brachial-femoral PWV; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, atherosclerosis, excluding cerebral, coronary, and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; GWAS, genome-wide association study; UKB: United Kingdom Biobank.
Results
The F-statistic for each SNP was greater than 10, which avoided the influence of weak IVs on our result to some extent. The integrated information of SNPs included for exposures is listed in Table 2, and the details of the characteristics of each SNP are shown in Table S1. SNPs for CPAmax in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S2. SNPs for CPSmax in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S3. SNPs for PAD in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S4. SNPs for PAD_FinnGen in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S5. SNPs for coronary artery atherosclerosis_FinnGen in the MR analyses: Harmonized Data (P < 5e-8, r2 < 0.001), related to Table 2, Table S6. SNPs for coronary atherosclerosis_FinnGen in the MR analyses: Harmonized Data (P < 5e-8, r2 < 0.001), related to Table 2, Table S7. SNPs for cerebral atherosclerosis_FinnGen in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S8. SNPs for atherosclerosis, excluding cerebral, coronary and PAD_FinnGen in the MR analyses: Harmonized Data (P < 5e-8, r2 < 0.001), related to Table 2, Table S9. SNPs for baPWV in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S10. SNPs for bfPWV in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S11. SNPs for pwASI in the MR analyses: Harmonized Data (P < 1e-5, r2 < 0.001), related to Table 2, Table S12. Causal effect of CPAmax on the risk of dementia, related to Figure 2, Table S13. Causal effect of CPSmax on the risk of dementia, related to Figure 2, Table S14. Causal effect of PAD on the risk of dementia, related to Figure 2, Table S15. Causal effect of PAD_FinnGen on the risk of dementia, related to Figure 2, Table S16. Causal effect of coronary artery atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S17. Causal effect of coronary atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S18. Causal effect of cerebral atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S19. Causal effect of atherosclerosis, excluding cerebral, coronary and PAD_FinnGen on the risk of dementia, related to Figure 2, Table S20. Causal effect of baPWV on the risk of dementia, related to Figure 2, Table S21. Causal effect of bfPWV on the risk of dementia, related to Figure 2, Table S22. Causal effect of pwASI on the risk of dementia, related to Figure 2. The integrated primary MR results obtained based on the IVW method are shown in Figure 2, and detailed results can be found in Table S12. Causal effect of CPAmax on the risk of dementia, related to Figure 2, Table S13. Causal effect of CPSmax on the risk of dementia, related to Figure 2, Table S14. Causal effect of PAD on the risk of dementia, related to Figure 2, Table S15. Causal effect of PAD_FinnGen on the risk of dementia, related to Figure 2, Table S16. Causal effect of coronary artery atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S17. Causal effect of coronary atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S18. Causal effect of cerebral atherosclerosis_FinnGen on the risk of dementia, related to Figure 2, Table S19. Causal effect of atherosclerosis, excluding cerebral, coronary and PAD_FinnGen on the risk of dementia, related to Figure 2, Table S20. Causal effect of baPWV on the risk of dementia, related to Figure 2, Table S21. Causal effect of bfPWV on the risk of dementia, related to Figure 2, Table S22. Causal effect of pwASI on the risk of dementia, related to Figure 2.
Table 2.
Integrated information of SNPs used for the two-sample Mendelian randomization analysis
| AD (ieu-b-2) |
AD (FinnGen) |
DLB (ebi-a-GCST90001390) |
VaD (FinnGen) |
FTD (ieu-b-43) |
FTD (meta) |
FTD (FinnGen) |
Any dementia (FinnGen) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nSNP | F | nSNP | F | nSNP | F | nSNP | F | nSNP | F | nSNP | F | nSNP | F | nSNP | F | |
| CPAmax | 6 | 135.529 | 6 | 133.772 | 3 | 62.505 | 6 | 133.772 | 6 | 133.772 | – | – | – | – | 6 | 133.772 |
| CPSmax | 10 | 221.629 | 9 | 199.484 | 9 | 198.063 | 9 | 199.484 | – | – | 6 | 128.390 | 9 | 199.484 | 9 | 199.484 |
| baPWV | 13 | 276.912 | 20 | 430.389 | 15 | 318.223 | 20 | 430.389 | – | – | 5 | 101.569 | 20 | 430.389 | 20 | 430.389 |
| bfPWV | 22 | 462.772 | 24 | 502.480 | 21 | 437.013 | 24 | 502.480 | 2 | 41.712 | 14 | 288.321 | 24 | 502.480 | 24 | 502.480 |
| PAD | 21 | 466.411 | 28 | 620.504 | 32 | 689.841 | 25 | 560.756 | 4 | 79.885 | 17 | 381.185 | 28 | 620.504 | 32 | 747.728 |
| PAD_FinnGen | 32 | 764.484 | – | – | 31 | 792.026 | – | – | 2 | 44.953 | 20 | 487.140 | – | – | – | – |
| pwASI | 27 | 604.263 | 31 | 749.774 | 35 | 811.501 | 34 | 818.562 | 3 | 68.256 | 23 | 512.824 | 36 | 863.547 | 30 | 668.411 |
| CoAS_FinnGen | 46 | 2600.420 | – | – | 44 | 2356.869 | – | – | 5 | 316.476 | 33 | 1743.254 | – | – | – | – |
| CeAS_FinnGen | 9 | 185.995 | – | – | 8 | 162.629 | – | – | 1 | 21.147 | 3 | 60.989 | – | – | – | – |
| ATHSCLE_FinnGen | 9 | 386.684 | – | – | 12 | 589.810 | – | – | 4 | 90.708 | 8 | 361.674 | – | – | – | – |
| CAD | 51 | 4023.882 | 58 | 4335.247 | – | – | 61 | 4514.494 | 8 | 558.928 | 60 | 4169.708 | 61 | 4514.494 | 56 | 4230.495 |
SNP, single nucleotide polymorphism; CPAmax, area of the largest carotid plaque detected; CPSmax, maximal degree of stenosis; PWV: pulse wave velocity; baPWV, brachial-ankle PWV; bfPWV, brachial-femoral PWV; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, Atherosclerosis, excluding cerebral, coronary and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; GWAS, genome-wide association study.
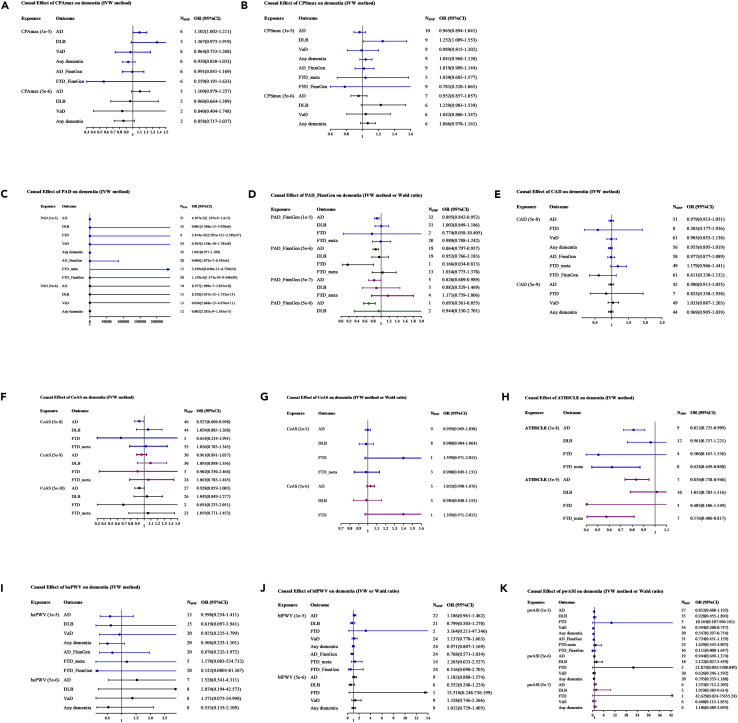
Figure 2.
Main results (IVW method) of the causal effect of atherosclerosis indicators on dementia
The causal effect of different atherosclerosis indicators on dementia subtypes was expressed as OR per unit. Error bars represent the 95% CIs of the estimates.
(A) The causal effect of CPAmax on dementia.
(B) The causal effect of CPSmax on dementia.
(C) The causal effect of PAD on dementia.
(D) The causal effect of PAD_FinnGen on dementia.
(E) The causal effect of CAD on dementia.
(F) The causal effect of CoAS on dementia.
(G) The causal effect of CeAS on dementia.
(H) The causal effect of ATHSCLE on dementia.
(I) The causal effect of baPWV on dementia.
(J) The causal effect of bfPWV on dementia.
(K) The causal effect of pwASI on dementia.
SNP, single nucleotide polymorphism; CPAmax, area of the largest carotid plaque detected; CPSmax, maximal degree of stenosis; PWV: pulse wave velocity; baPWV, brachial-ankle PWV; bfPWV, brachial-femoral PWV; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, atherosclerosis, excluding cerebral, coronary, and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; IVW, inverse variance weighted; OR, odd ratio; CI, confidence interval.
Causal effect of CPAmax and CPSmax on the risk of dementia via initial and sensitivity analyses
A total of 3–6 independent SNPs for CPAmax and 5–10 independent SNPs for CPSmax were selected as IVs and the F-statistics ranged from 19.789 to 28.836 (selection criterion, 1e-5). After correction for multiple tests (STAR methods), suggestive evidence from the initial IVW analyses showed that genetically predicted CPAmax was positively associated with the development of AD (OR = 1.102, 95% CI: 1.002–1.211, p = 0.045), while genetically predicted CPSmax was positively associated with the development of DLB (OR = 1.252, 95% CI, 1.009–1.553, p = 0.041). Although no significant results were obtained by other sensitivity MR methods, the effect directions were all consistent with the main IVW method (Figures 2A and 2B, Tables S12 and S13). Notably, the aforementioned suggestive positive causal associations were no longer present when a more stringent selection criterion (5e-6) was applied. Through Phenoscanner retrieval, no significant correlation was found between the excluded SNPs (CPAmax: rs72942431, rs2076835, and rs150656634; CPSmax: rs1149250, rs2076835, and rs12985814) and other traits. All models suggested that CPAmax had no causal effect on the risk of DLB (ORIVW = 1.367, 95% CI: 0.973–1.919, p = 0.071), VaD (ORIVW = 0.964, 95% CI: 0.733–1.268, p = 0.794), and any dementia (ORIVW = 0.930, 95% CI: 0.838–1.033, p = 0.177). Since no overlapping SNPs could be screened, the causal association between CPAmax and FTD/FTD_meta could not be analyzed. We conducted additional sensitivity analysis using FTD and AD data from the FinnGen consortium (STAR methods), and the results showed that no evidence was detected for a causal relation between CPAmax and FTD_FinnGen (ORIVW = 0.559, 95% CI: 0.191–1.633, p = 0.288) or AD_FinnGen (ORIVW = 0.991, 95% CI: 0.841–1.169, p = 0.916) (Table S11).
All models suggested that CPSmax had no causal effect on the risk of AD (ORIVW = 0.965, 95% CI: 0.894–1.041, p = 0.356), VaD (ORIVW = 0.989, 95% CI: 0.815–1.202, p = 0.918), and any dementia (ORIVW = 1.041, 95% CI: 0.968–1.120, p = 0.278). Similarly, due to the inability to screen out overlapping SNPs in CPSmax and FTD, we conducted supplementary analysis on the causal relationship between CPSmax and FTD_meta as well as FTD_FinnGen and did not find any causal relationship between them (Table S12).
Causal effect of PAD and CAD on the risk of dementia via initial and sensitivity analyses
In the initial analysis of PAD, 4–32 SNPs were selected and the F-statistics ranged from 19.516–68.505 (selection criterion, 1e-5). All MR models consistently showed that there was no causal effect of PAD on the risk of any dementia subtype (Figure 2C and Table S14). The results were consistent when stricter criteria for IV selection (5e-6) were adopted (STAR methods).
In sensitivity analysis, a total of 2–32 independent SNPs for PAD_FinnGen were selected as IV, and the F-statistics ranged from 19.516–89.696 (selection criterion, 1e-5). The IVW analysis indicated that genetically determined PAD_FinnGen (OR: 0.895, 95% CI: 0.842–0.952, p = 0.0004) was reversely associated with the risk of AD (Figure 2D). Although no significant results were obtained by other MR models except the weighted median method (suggestive evidence, OR (95% CI): 0.875(0.797–0.962), p = 0.006), the effect direction was consistent with the main IVW method. Even if the screening criteria for IVs are strict to 5e-8, 5e-7, or 5e-6 (STAR methods), the reverse causal relationship between PAD_FinnGen and AD still has significant or suggestive significance (OR5e-8: 0.693, 95% CI: 0.561–0.855, p = 0.0006; OR5e-7: 0.826, 95% CI: 0.689–0.989, p = 0.038; OR5e-6: 0.864, 95% CI: 0.797–0.937, p = 0.0004). Several excluded SNPs were significantly associated with systemic sclerosis (rs145659637), coronary artery disease (rs117733303 and rs2107595), sitting height (rs60583357), and lipid metabolism (rs17248727, rs145947882, and rs2980888). All models suggested that PAD_FinnGen has no causal effect on the risk of DLB (ORIVW = 1.003, 95% CI: 0.849–1.186, p = 0.968). Since only 2 SNPs were selected as IV to estimate the causal effect of PAD_FinnGen on the FTD risk, we performed a supplementary sensitivity analysis using another larger FTD summary dataset and the MR result (PAD_FinnGen to FTD_meta) was consistent with the initial result (Table S15).
A total of 8–61 independent SNPs for CAD were selected, and the F-statistics ranged from 29.825 to 595.221 (selection criterion, 5e-8). All MR models consistently showed that there was no causal effect of CAD on the risk of any dementia subtype (Figure 2E). The results were consistent when stricter criteria for IV selection (5e-9) were adopted (Table S16).
Causal effect of CoAS, CeAS, and ATHSCLE on the risk of dementia via initial and sensitivity analyses
A total of 5–46 independent SNPs for CoAS were selected as IVs, and the F-statistics ranged from 29.928 to 209.929 (selection criterion, 5e-8). After correction for multiple tests (STAR methods), suggestive evidence from the initial IVW analyses showed that genetically predicted CoAS was inversely associated with the development of AD (OR = 0.927, 95% CI: 0.860–0.998, p = 0.045). Although no significant results were obtained by other sensitivity MR methods, the effect directions were all consistent with the main IVW method (Figure 2F, Tables S17). Notably, the aforementioned reverse causation was no longer present when a more stringent selection criterion (5e-9 and 5e-10) was applied. Through phenoscanner query, several excluded SNPs were significantly related to coronary artery disease (rs10774624, rs11670056, rs16986953, rs2246828, rs3776299, and rs72938351), diabetes or height (rs3134943 and rs558797786), and diastolic blood pressure (rs665834).
A total of 1–9 independent SNPs for CeAS were selected, and the F-statistics ranged from 19.556 to 21.946 (selection criterion, 1e-5). All MR models consistently showed that there was no causal effect of CeAS on the risk of any dementia subtype (Figure 2G). The results were consistent when stricter criteria for IV selection (5e-6) were adopted (Table S18).
A total of 8–12 independent SNPs for ATHSCLE were selected as IVs (the F-statistics ranged from 19.702 to 108.725 (selection criterion, 5e-8)) to estimate its effect on the risk of AD, DLB, and FTD_meta. After correction for multiple tests, suggestive evidence from the initial IVW analyses showed that genetically predicted ATHSCLE was inversely associated with the development of AD (OR = 0.812, 95% CI: 0.725–0.909, p = 0.0003) and FTD_meta (OR = 0.624, 95% CI: 0.449–0.868, p = 0.005). Although no significant results were obtained by other sensitivity MR methods, the effect directions were all consistent with the main IVW method (Figure 2H). Notably, the aforementioned reverse causation persisted when a more stringent selection criterion (5e-9) was applied (Table S19), and the excluded SNPs (rs2273500 and rs35236974) were significantly related to smoking. Due to the absence of IVs that satisfy 5e-8 for estimating the causality between ATHSCLE and FTD, two less stringent choices (1e-5 and 5e-6) were used (STAR methods). The consistent results indicated that there was no causal relationship between ATHSCLE and FTD.
Causal effect of baPWV and bfPWV on the risk of dementia via initial and sensitivity analyses
A total of 5–20 independent SNPs for baPWV and 2–24 independent SNPs for bfPWV were selected as IV and the F-statistics ranged from 19.618 to 28.441 (selection criterion, 1e-5). All models in the initial and supplementary sensitivity analyses consistently suggested no causal relationship between baPWV or bfPWV and any dementia subtype (Figures 2I and 2J), and there was also no evidence of heterogeneity and pleiotropy effect based on Cochran’s Q and MR-Egger intercept tests (Tables S20 and S21).
Causal effect of pwASI on the risk of dementia via initial and sensitivity analyses
In the initial analysis, 3–35 SNPs were selected and the F-statistics ranged from 19.709 to 64.902 (selection criterion, 1e-5). All MR models consistently showed that there was no causal effect of pwASI on the risk of AD, DLB, and FTD (Figure 2K, Table S22). After correction for multiple tests (STAR methods), suggestive evidence from the initial IVW analyses showed that genetically predicted pwASI was reversely associated with the development of VaD (OR = 0.394, 95% CI: 0.208–0.747, p = 0.004), while genetically predicted pwASI was significantly associated with the development of any dementia (OR = 0.547, 95% CI, 0.397–0.754, p = 0.0002). Although no significant results were obtained by other sensitivity MR methods, the effect directions were all consistent with the main IVW method. Notably, the previously suggestive reverse causations were no longer present when more stringent selection criteria (5e-6 and 5e-7) were applied (Table S22). Through phenoscanner query, the excluded SNPs were significantly associated with height or BMI (rs1111088, rs9501489, and rs11513729), coronary artery disease, or lipid metabolism (rs651007 and rs11513729).
In sensitivity analysis (STAR methods), a total of 23–36 independent SNPs for pwASI were selected as IV, and the F-statistics ranged from 19.709 to 64.902. All models suggested that pwASI has no causal effect on the risk of FTD_meta, FTD_FinnGen, and AD_FinnGen (Table S22).
In general, the sensitivity analyses using different analytical methods confirmed the reliability of our results (STAR methods). First, no heterogeneity or horizontal pleiotropy was detected using Cochran’s Q, MR-Egger intercept, and MR-PRESSO tests. Second, all leave-one-out plots showed that no single SNP drove the causal estimates (Figure 3 and Figures S1–S95).
Figure 3.
MR leave-one-out sensitivity analysis of the effect of PAD_FinnGen and ATHSCLE_FinnGen on AD or FTD_meta
Each row represents an MR analysis of the effect of PAD_FinnGen on AD and ATHSCLE_FinnGen on AD and FTD_meta, using all instruments except for the SNP associated with exposure listed on the y axis. The point represents the beta with that SNP removed, and the line represents the 95% confidence interval. The screening thresholds for PAD_FinnGen were 1e-5 (A1), 5e-6 (A2), and 5e-7 (A3), and the screening thresholds for ATHSCLE_FinnGen were 5e-8 (B1 and C1) and 5e-9 (B2 and C2). PAD, peripheral artery disease; ATHSCLE, atherosclerosis, excluding cerebral, coronary, and PAD; AD, Alzheimer’s disease; FTD, frontotemporal dementia.
Discussion
We conducted conventional two-sample MR analyses to estimate the putative causal effect of subclinical atherosclerosis indicators or arterial stiffness, including CPAmax, CPSmax, PAD, CAD, CoAS, CeAS, ATHSCLE, baPWV, bfPWV, and pwASI, on the risk of various dementia subtypes. Contrary to previous studies, we found suggestive evidence supporting a reverse causal effect of PAD_FinnGen, CoAS, and ATHSCLE on AD risk and a reverse causal effect of ATHSCLE on FTD risk. Although the initial analysis of PAD and AD did not yield consistent results with the sensitivity analysis, this finding is still unexpected and requires further exploration. The suggestive evidence of adverse effects of CPAmax and CPSmax on the risk of AD and DLB, as well as the significant evidence of a positive effect of pwASI on the risk of VaD and any dementia, are basically consistent with published observational studies but no longer persisted after more stringent selection criteria for IV were used. Unexpectedly, we did not find any evidence in support of a causal effect of PWV on the risk of any dementia subtype. Although dementia subtypes are highly correlated, the pathological mechanisms of AD and other dementia subtypes are not identical,23 which is one of the reasons why the causal effects of the same atherosclerosis indicators on different dementia subtypes are inconsistent. Similarly, different arterial stiffness and subclinical atherosclerotic indicators reflect different pathological lesions of arterial vessels, so their effects on the same dementia subtype may be different.
Atherosclerosis is a chronic inflammation of the vessel commonly found in the carotid artery, and subclinical atherosclerosis is considered a potential risk factor for cognitive impairment and progression to dementia, with most of the evidence originating from European, African, or East Asian populations.19,20,21 Although vascular pathologies are also a critical component of AD,27 the correlation between carotid atherosclerosis and dementia is still controversial.6,28,29 Carotid artery plaque and stenosis are established markers of subclinical atherosclerosis, and the degree of stenosis is significantly related to cognitive decline.30 Using representative indicators (CPAmax and CPSmax) reflecting the characteristics of arteriosclerosis described previously, this MR study found a suggestive positive causal effect of CPAmax (area of the largest carotid plaque) on the risk of AD as well as CPSmax (degree of carotid stenosis) on DLB risk, which was in line with recently published results in a cross-sectional study.20 The disappearance of significance after adopting more stringent IV selection criteria and the insignificant findings of the association between CPSmax and AD risk as well as these two indicators and other dementia subtypes may be related to the following factors: (1) Fewer IVs are more likely to result in false negative results due to insufficient power.31 (2) The specific location of carotid stenosis. Based on the findings of Fergenbaum JH et al.,32 bilateral or left carotid artery stenosis rather than right carotid artery stenosis was associated with decreased cognitive ability. (3) The sex and age ratio of the sample in summary data. Atherosclerosis is known for its sexual dimorphism, and men exhibit a higher risk than women, although the underlying mechanisms are only partly understood.33 In addition, advanced age is a well-known risk factor for cognitive impairment.34 Therefore, summary data with more sample and detailed stratified information should be developed for analysis in the future.
It is widely accepted that peripheral atherosclerosis may deteriorate neurodegenerative processes through endothelial cell dysfunction and microvascular impairment.35 Peripheral artery disease (PAD), characterized by an ankle-to-brachial index (ABI) < 0.90 or >1.40, is a manifestation of underlying generalized atherosclerosis.36 Several epidemiological studies have shown that lower ABI (increased odds of PAD) may contribute to the aggravation of AD-related cognitive impairment and VaD,13,18,37 suggesting the presence of a relationship between atherosclerosis of cerebral arteries and dementia and cognitive decline in older adults. Although no causal association between PAD and dementia was observed in our initial MR analysis, which may be related to the small sample size,38 a suggestive reverse causality was detected in the sensitivity analysis. Similarly, the insignificant causality between CeAS and dementia may also be due to the limited sample size. From another perspective, some studies reported that both high and low ABI were positively related to an increased risk of dementia, particularly a U-shaped relationship.18 Additionally, the Ankle Brachial Index Collaboration demonstrated a reverse J-shaped relationship of ABI with mortality and coronary events with a low risk ABI ranging from 1.11 to 1.40.36 The complex mechanisms mentioned previously, as well as the potential differences in the definition of PAD among different databases, may be other possible reasons for the insignificant and inconsistent results of the initial and sensitivity analyses. In addition, the lower extremity PAD burden differs by race/ethnicity. Even if we selected summary data that primarily focus on European populations, the database that aggregates data from multiple consortia does not rule out the possibility of the presence of some other ethnic individuals. Consistent with the result of a published MR study,39 our study did not find a causal effect of CAD on dementia, which indicated that the treatment of CAD may not directly prevent dementia, but the indirect beneficial effect of CAD prevention measures on dementia risk cannot be ruled out. Since atherosclerosis and dementia are both age-related diseases with similar risk factors (smoking, hypertension, diabetes, dyslipidemia, etc.),40 which have been verified by the Phenoscanner V2 tool, the suggestive reverse causal effect of CoAS and ATHSCLE on the AD risk and the suggestive reverse causal effect of ATHSCLE on the FTD risk observed in this study need careful interpretation and further exploration. Even so, these results suggested that current lifestyle interventions, preventive measures, and treatment strategies recommended for atherosclerosis may have indirect benefits in reducing dementia risk.
Arterial stiffness, another independent factor for dementia,41 increases before any visible changes appear in the vascular wall structure and is a precursor to the development of atherosclerosis. PWV is an integrative marker of arterial function and is regarded as the gold-standard noninvasive measure of arterial stiffness.42 One previous MR study42 confirmed a bidirectional causal effect of PWV and blood pressure, which is a risk factor for dementia. It is plausible that PWV should be positively correlated with the progressive decline in cognitive function.20 However, no causal association of baPWV and bfPWV with the risk of any dementia was detected in our MR study, which is consistent with the results of a prospective study, even if different PWV modes were used.29 Given the strong positive correlations between cfPWV and baPWV as well as between cfPWV and bfPWV,43 and the limited IV of cfPWV (only one SNP), only baPWV and bfPWV were included in the MR analysis. Several possible pathophysiological mechanisms may contribute to the uncorrelated association between PWV and cognitive function. First, a previous study20 found a significant association between higher PWV and greater cognitive decline in specific cognitive domains but did not provide evidence for an association with global cognitive function. Second, arterial stiffness can be divided into two mechanisms (load-dependent and structural stiffening), and total stiffness was associated with dementia but load-dependent and structural stiffness were not.44 Third, baPWV is strongly correlated with age and body mass index (BMI), which may be a key factor in causal inference.43,45 Of note, the current study found pwASI, another marker of arterial stiffness, significantly associated with any dementia, and suggestively associated with VaD, but the causality was no longer present when more stringent screening criteria were used. Using the Phenoscanner tool, it can be seen that the excluded SNPs are strongly correlated with height, sitting height, BMI, and even hypertension, hyperlipidemia, and hyperglycemia, confirming the possibility of the impact of pleiotropic SNPs on false positive results in the original analysis. The ASI was developed to evaluate arterial stiffness based on an analysis of the pulse wave amplitude pattern acquired from measurements of brachial blood pressure.46 In addition to the potential reasons mentioned previously, the methodological differences between arterial stiffness measurement and pulse wave velocity may be another consideration.47 Ultimately, the results should be interpreted with caution, and further studies are needed to explore any potential association of arterial stiffness and dementia.
There are several advantages of our study. First, we used a two-sample MR approach to explore the causal relationship between various atherosclerosis indicators and different types of dementia as comprehensively as possible. Second, to improve the reliability of our results, different GWAS datasets and IV screening criteria were used, and multiple analytical methods were conducted. Finally, although no valid evidence was found, the analytical thinking of exploring the causality between subclinical indicators, that is, disease detected noninvasively before it produces clinical signs and symptoms, and outcomes may have certain reference value.
Limitations of the study
Inevitably, there are also several limitations to this study. First, due to the limited GWAS data available, we only used six arterial stiffness or atherosclerosis indicators and four representative types of atherosclerosis for analysis. The summary statistics of several indicators (i.e., ABI or intima-media thickness (IMT)) cannot be obtained, and all included indicators except PAD lack another database for sensitivity analysis. Second, the small sample size of exposures and outcomes may be a major factor affecting the reliability of the MR results. By calculating the F-statistic, even if there are no weak IVs, the MR results should be interpreted with caution. Meanwhile, GWAS with a larger sample size also needs further development. Third, as the relevant GWAS research is mainly conducted among participants of European descent, further research is needed to assess the universality of our results among other ethnic groups. Last but not least, although arterial stiffness and atherosclerosis were observed causal relationships with different subtypes of dementia to varying degrees, this study was still unable to determine their priority in the risk of dementia. In addition, Celelja M et al.48 indicated that the changes of atherosclerosis in the arterial wall were not necessarily related to arterial stiffness, so the association between arterial stiffness and atherosclerosis as well as their mechanisms on the development of dementia needs to be further explored.
In this work, we sought to advance our understanding of the causal relationship between arterial stiffness or subclinical atherosclerosis indicators and dementia risk. We identified a suggestive reverse causal effect of PAD, CoAS, and ATHSCLE on AD, and a suggestive reverse causal effect of ATHSCLE on FTD. The Phenoscanner query results suggested that the disappearance of the significance results in the original analysis might be attributed to those pleiotropic SNPs. In addition, subclinical atherosclerosis indicators are modifiable, and their reliable association with dementia means that many subtypes of dementia may be prevented or delayed. Therefore, GWASs with larger sample size are needed for further research to identify which subclinical atherosclerosis indicators can better predict cognitive decline or dementia.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| GWAS summary statistics for CPAmax and CPSmax | Pott et al.33 | https://www.health-atlas.de/ |
| GWAS summary statistics for PAD | IEU OpenGWAS project | https://gwas.mrcieu.ac.uk/ |
| GWAS summary statistics for PAD_FinnGen | FinnGen consortium | https://www.finngen.fi/en |
| GWAS summary statistics for CAD | IEU OpenGWAS project | https://gwas.mrcieu.ac.uk/ |
| GWAS summary statistics for CoAS, CeAS and ATHSCLE | FinnGen consortium | https://www.finngen.fi/en |
| GWAS summary statistics for baPWV and bfPWV | Rode et al.49 | https://www.health-atlas.de/ |
| GWAS summary statistics for pwASI | IEU OpenGWAS project | https://gwas.mrcieu.ac.uk/ |
| Software and algorithms | ||
| R | https://www.r-project.org | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Youxin Wang (wangy@ccmu.edu.cn).
Materials availability
The study did not generate any new materials.
Data and code availability
This paper analyzes existing, publicly available data. These accession websites for the datasets are listed in the key resources table. The original code is available in this paper’s supplemental information (Methods S1). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Ethics statement
Publicly available GWAS summary data were used in this MR study, so no additional ethical approval was required.
Study design
Our study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR)50 guidelines for transparent reporting of MR analyses.
The MR analysis is based on the following three core hypotheses51: 1) the instrumental variable (IV) should be robustly associated with the exposure (atherosclerotic indicators). The independent (linkage disequilibrium (LD), r2 < 0.001 within a 10,000 kb window) single nucleotide polymorphism (SNP) that satisfied the GWAS significance (p < 5 × 10−8) or relaxed threshold (p < 1 × 10−5) referring to published studies52,53 (in the case of a limited number of SNPs) was selected as IV, and the F-statistic (F = beta2/se2) was calculated to assess the strength of each genetic instrument.54 A higher F-statistic indicates a lower risk of weak IV bias. 2) The IV is independent of confounding factors. To minimize the possibility of confounders caused by population stratification, all data sources were limited to European individuals. 3) The IV influences the outcome (dementia) exclusively via the exposure.
GWAS data for atherosclerotic indicators
Data on the area of the largest carotid plaque detected (CPAmax) and maximal degree of stenosis (CPSmax) were derived from the LIFE-Adult cohort (N = 727 men and 550 women).33 The PWV summary statistics were obtained from a GWAS for three PWV modes, namely, cfPWV, brachial-ankle PWV (baPWV) and brachial-femoral PWV (bfPWV), in the LIFE-Adult study (N = 3,643-6,734).49 Summary statistics of PAD (GWAS ID: ukb-d-I9_PAD 55) and pulse wave arterial stiffness index (pwASI) (GWAS ID: ukb-b-11971, MRC-IEU Consortium56) were obtained from the United Kingdom Biobank (UKB), which enrolled a total of 362,194 samples (9,637,467 SNPs) for PAD and 151,053 individuals (9,851,867 SNPs) for pwASI. As a sensitivity analysis, additional summary data on PAD (phenocode: I9_PAD) with more PAD cases (9,021 cases and 244,907 controls) derived from the FinnGen consortium57 (release 6, accessed on 24 April 2023) were used to explore its causal effect on dementia from non-FinnGen data. Summary data on coronary atherosclerosis, cerebral atherosclerosis and atherosclerosis (excluding cerebral, coronary and PAD) were also obtained from the FinnGen consortium (release 8).57 The coronary artery disease (CAD) summary statistics58 (GWAS ID: ebi-a-GCST9005195) were derived from the datasets that satisfy minimum requirements imported from the EBI database of complete GWAS summary data.
GWAS data for dementia
The summary data on AD (GWAS ID: ieu-b-2, sample size: 21,982 cases and 41,944 controls)59 and FTD (GWAS ID: ieu-b-43, sample size: 515 cases and 2,509 controls)60 were obtained from the recently published GWAS, which included many different consortia of participants with European ancestry. AD is the most common dementia subtype, and FTD is commonly underdiagnosed because it is a heterogeneous spectrum. As an additional sensitivity analysis, we utilized another publicly available GWAS summary statistic for AD57 (phenocode: F5_ALZHDEMENT, sample size: 4,406 cases and 325,306 controls) and FTD (FTD_meta26: 2,154 cases and 4,308 controls; FTD_FinnGen57: 99 cases and 328,323 controls). A detailed description of these GWAS summary statistics is available in the corresponding publication. The summary data on DLB (GWAS ID: ebi-a-GCST900001390, sample size: 2,591 cases and 4,027 controls)61 were obtained from the datasets that satisfy minimum requirements imported from the EBI database of complete GWAS summary data. Vascular dementia (phenocode: F5_VASCDEM, sample size: 2,048 cases/328,982 controls) and any dementia (phenocode: KRA_PSY_DEMENTIA_EXMORE, sample size: 12,042 cases/254,976 controls) were both derived from the FinnGen database57 (release 8, accessed on 24 April 2023).
Method details
MR analysis
The fixed-effect inverse-variance weighted (IVW)62 analysis was used as the primary method, which applies a meta-analysis approach to integrate the Wald ratio of each SNP. To ensure that the three core assumptions were not breached, MR-Egger regression,63,64 weighted median,65 and weighted mode66 methods were performed as sensitivity analysis methods. These three robust methods can produce a valid estimate of the causal relationship under different assumptions.
Sensitivity analysis
Heterogeneity was assessed by Cochran’s Q heterogeneity test in the IVW method (the Q-statistic p < 0.05 indicated the presence of heterogeneity), and outlier SNPs were detected and excluded by the Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) method. If MR-PRESSO failed to detect outliers, then IVW radial regression and Egger radial regression were implemented.67,68 The intercept test (p > 0.05 means no pleiotropy) of MR-Egger regression was used to assess horizontal pleiotropy. MR-PRESSO analysis was used to detect (global test) and correct (outlier test) and remove (distortion test) the abnormal SNPs.67 We conducted leave-one-out analysis to explore the influence of each genetic variant on the outcome.69
A common statistical method when selecting genetic variations is to include all variations related to exposure at the genome-wide significance threshold level (p < 5 × 10−8, a stringent level that was used to control the number of false positive findings).31 If such variants are not available, we can consider using a less stringent choice of variants (5e-7, 5e-6, 1e-5, etc.), which generally have no single correct criterion as long as they do not violate the instrumental variable assumption. Considering the reliability of MR results, we performed sensitivity analysis using different SNP screening thresholds (especially for exposures with 2-digit SNPs and non-null results) and summary datasets released from other available sources, provided that these datasets meet the requirements of two-sample MR, that is, the exposure and outcome datasets come from two independent non-overlapping samples of a homogeneous population.70 In addition, the Phenoscanner V271,72 tool (http://www.phenoscanner.medschl.cam.ac.uk/) was used to check whether the excluded SNPs were strongly related to diseases or phenotypes other than exposures, known as horizontal pleiotropy.
Qualification and statistical analysis
Statistics and software
Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) of dementia correspond to dementia risk per standard deviation (SD) increase in log odds of the atherosclerosis indicators. In this study, we included analyses of 11 atherosclerotic indicators and 8 subtypes of dementia. Considering multiple testing and referring to previous MR studies,73,74,75 we used a conservative method and applied a Bonferroni corrected significance level computed as 0.05 divided by 88 (that is, α = 0.05/11/8 = 0.0006). p < 0.0006 was considered strong evidence of a causal association, while p < 0.05 but above the Bonferroni corrected significance threshold (0.0006) was considered suggestive of evidence for a potential association. All data analyses were performed using R software (version 4.1.2, https://www.r-project.org/), and MR analyses were performed using the "TwoSampleMR" and "MR-PRESSO" packages.
Acknowledgments
This work was supported by the National Key R&D Program of China (grant number 2017YFE0118800). Funders had no role in the design and conduct of the study, the collection and analysis of the data, and the writing and submission of the manuscript.
The authors want to acknowledge the participants and investigators of the FinnGen study and all the consortia for providing the GWAS datasets available to the public.
Author contributions
Conceptualization: Y.W. and S.W. Methodology: Q.Z. and X.Z. Software: Q.Z. Validation: Y.W. and S.W. Formal analysis: Q.Z. and Y.W. Investigation: Q.Z. and G.W. Resources: Q.Z. and G.W. Data curation: Q.Z. and Y.W. Writing – original draft: Q.Z. Writing.–.review & editing: Y.W. and S.W. Visualization: Q.Z. and G.W. Supervision: Y.W. and S.W. Project administration: Y.W. and Q.Z. Funding acquisition: Y.W.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: October 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108325.
Contributor Information
Sheng Wang, Email: shengwang@mail.ccmu.edu.cn.
Youxin Wang, Email: wangy@ccmu.edu.cn.
Supplemental information
SNP, single-nucleotide polymorphism; CHR, chromosome; POS, position; OA, other_allele; EA, effect_allele; SE, standard error; CPAmax, area of the largest carotid plaque detected; CPSmax, maximal degree of stenosis; PWV, pulse wave velocity; baPWV, brachial-ankle PWV; bfPWV, brachial-femoral PWV; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, atherosclerosis, excluding cerebral, coronary and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; MR, Mendelian randomization; F-statistic = Beta2/SE2; IVW, inverse variance weighted; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; OR, odd ratio; CI, confidence interval.
References
- 1.Zheng J., Ni C., Zhang Y., Huang J., Hukportie D.N., Liang B., Tang S. Association of regular glucosamine use with incident dementia: evidence from a longitudinal cohort and Mendelian randomization study. BMC Med. 2023;21:114. doi: 10.1186/s12916-023-02816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nassan M., Daghlas I., Piras I.S., Rogalski E., Reus L.M., Pijnenburg Y., Cuddy L.K., Saxena R., Mesulam M.M., Huentelman M. Evaluating the association between genetically proxied ACE inhibition and dementias. Alzheimers Dement. 2023;19:3894–3901. doi: 10.1002/alz.13062. [DOI] [PubMed] [Google Scholar]
- 3.Li H., Chen K., Yang L., Wang Q., Zhang J., He J. The role of plasma cortisol in dementia, epilepsy, and multiple sclerosis: A Mendelian randomization study. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X.M., Liu J., Cao M., Yang T.T., Wang Y.Q., Hou Y.L., Song Q., Cui Y.T., Wang P.C. TREM2: A Novel Potential Biomarker of Alzheimer's Disease. Biomed. Environ. Sci. 2021;34:719–724. doi: 10.3967/bes2021.099. [DOI] [PubMed] [Google Scholar]
- 6.van Oijen M., de Jong F.J., Witteman J.C.M., Hofman A., Koudstaal P.J., Breteler M.M.B. Atherosclerosis and risk for dementia. Ann. Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart S.N., Schaich C.L., Craft S., Sachs B.C., Rapp S.R., Jung Y., Whitlow C.T., Solingapuram Sai K.K., Cleveland M., Williams B.J., et al. Associations among vascular risk factors, neuroimaging biomarkers, and cognition: Preliminary analyses from the Multi-Ethnic Study of Atherosclerosis (MESA) Alzheimers Dement. 2022;18:551–560. doi: 10.1002/alz.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lineback C.M., Stamm B., Sorond F., Caprio F.Z. Carotid disease, cognition, and aging: time to redefine asymptomatic disease? GeroScience. 2023;45:719–725. doi: 10.1007/s11357-022-00688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman R.F., Albert M.S., Alonso A., Coker L.H., Coresh J., Davis S.M., Deal J.A., McKhann G.M., Mosley T.H., Sharrett A.R., et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Norby F.L., George K.M., Alonso A., Mosley T.H., Gottesman R.F., Meyer M.L., Lutsey P.L. Association of Carotid Intima-Media Thickness and Other Carotid Ultrasound Features With Incident Dementia in the ARIC-NCS. J. Am. Heart Assoc. 2021;10 doi: 10.1161/jaha.120.020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangen K.J., Smirnov D.S., Delano-Wood L., Wierenga C.E., Bondi M.W., Salmon D.P., Galasko D. Arterial stiffening acts synergistically with APOE genotype and AD biomarker status to influence memory in older adults without dementia. Alzheimer's Res. Ther. 2021;13:121. doi: 10.1186/s13195-021-00851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Huang J., Wu T., Qi L. Arterial Stiffness, Genetic Risk, and Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care. 2022;45:957–964. doi: 10.2337/dc21-1921. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y., Xu M., Xie L., Wang T., Huang X., Lv X., Chen Y., Ding L., Lin L., Wang W., et al. Obesity and peripheral arterial disease: A Mendelian Randomization analysis. Atherosclerosis. 2016;247:218–224. doi: 10.1016/j.atherosclerosis.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Bis J.C., Kavousi M., Franceschini N., Isaacs A., Abecasis G.R., Schminke U., Post W.S., Smith A.V., Cupples L.A., Markus H.S., et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat. Genet. 2011;43:940–947. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschini N., Giambartolomei C., de Vries P.S., Finan C., Bis J.C., Huntley R.P., Lovering R.C., Tajuddin S.M., Winkler T.W., Graff M., et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat. Commun. 2018;9:5141. doi: 10.1038/s41467-018-07340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pott J., Burkhardt R., Beutner F., Horn K., Teren A., Kirsten H., Holdt L.M., Schuler G., Teupser D., Loeffler M., et al. Genome-wide meta-analysis identifies novel loci of plaque burden in carotid artery. Atherosclerosis. 2017;259:32–40. doi: 10.1016/j.atherosclerosis.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.J., Lee Y.N., Wu T.W., Chou C.L., Wang L.Y. Common Genetic Variants on Bone Morphogenetic Protein Receptor Type IB (BMPR1B) Gene Are Predictive for Carotid Intima-Media Thickness. Circ. J. 2019;83:749–756. doi: 10.1253/circj.CJ-18-1046. [DOI] [PubMed] [Google Scholar]
- 18.Moon S.W., Byun M.S., Yi D., Kim M.J., Jung J.H., Kong N., Jung G., Ahn H., Lee J.Y., Kang K.M., et al. Low Ankle-Brachial Index Relates to Alzheimer-Signature Cerebral Glucose Metabolism in Cognitively Impaired Older Adults. J. Alzheimers Dis. 2023;93:87–95. doi: 10.3233/jad-220911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vibha D., Prasad K., Dwivedi S.N., Kant S., Pandit A.K., Tiemeier H., Srivastava A.K., Karthikeyan G., Garg A., Verma V., et al. Carotid Intima-Media Thickness (cIMT) and Cognitive Performance: A Population-Based Cross-Sectional Study From North India. Alzheimer Dis. Assoc. Disord. 2023;37:35–41. doi: 10.1097/wad.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y., Zhu Y., Ma Y., Li C., Hua R., Zhong B., Wang H., Xie W. Association of subclinical atherosclerosis and cognitive decline: a community-based cross-sectional study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-059024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D., Huang Z., Dai Y., Guo L., Lin S., Liu X. Bioinformatic identification of potential biomarkers and therapeutic targets in carotid atherosclerosis and vascular dementia. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.1091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith E.J.T., Gasper W.J., Schneider P.A., Finlayson E., Walter L.C., Covinsky K.E., Conte M.S., Iannuzzi J.C. Cognitive Impairment is Common in a Veterans Affairs Population with Peripheral Arterial Disease. Ann. Vasc. Surg. 2023;91:210–217. doi: 10.1016/j.avsg.2022.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Yu X., Lophatananon A., Mekli K., Burns A., Muir K.R., Guo H. A suggested shared aetiology of dementia - a colocalization study. Neurobiol. Aging. 2022;117:71–82. doi: 10.1016/j.neurobiolaging.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Habenicht L.K.L., Wang Z., Zhang X., Li Y., Mogler C., Huspenina J.S., Schmid R.M., Weber C., Mohanta S.K., Ma Z., Yin C. The C1q-ApoE complex: A new hallmark pathology of viral hepatitis and nonalcoholic fatty liver disease. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.970938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emdin C.A., Khera A.V., Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari R., Hernandez D.G., Nalls M.A., Rohrer J.D., Ramasamy A., Kwok J.B.J., Dobson-Stone C., Brooks W.S., Schofield P.R., Halliday G.M., et al. Frontotemporal dementia and its subtypes: a genome-wide association study. Lancet Neurol. 2014;13:686–699. doi: 10.1016/s1474-4422(14)70065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bickel M.A., Csik B., Gulej R., Ungvari A., Nyul-Toth A., Conley S.M. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1087053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arntzen K.A., Schirmer H., Johnsen S.H., Wilsgaard T., Mathiesen E.B. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects - the Tromsø study. Cerebrovasc. Dis. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson E.D., Elmståhl S., Minthon L., Pihlsgård M., Nilsson P.M., Hansson O., Nägga K. No independent association between pulse wave velocity and dementia: a population-based, prospective study. J. Hypertens. 2017;35:2462–2467. doi: 10.1097/hjh.0000000000001480. [DOI] [PubMed] [Google Scholar]
- 30.Li J., Wang Y.J., Zhang M., Xu Z.Q., Gao C.Y., Fang C.Q., Yan J.C., Zhou H.D., Chongqing Ageing Study Group Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 31.Burgess S., Thompson S.G. Chapman&Hall/CRC; 2015. Mendelian Randomization, Methods for Using Genetic Variants in Causal Estimation. [Google Scholar]
- 32.Fergenbaum J.H., Bruce S., Spence J.D., Lou W., Hanley A.J.G., Greenwood C., Young T.K. Carotid atherosclerosis and a reduced likelihood for lowered cognitive performance in a Canadian First Nations population. Neuroepidemiology. 2009;33:321–328. doi: 10.1159/000254294. [DOI] [PubMed] [Google Scholar]
- 33.Pott J., Beutner F., Horn K., Kirsten H., Olischer K., Wirkner K., Loeffler M., Scholz M. Genome-wide analysis of carotid plaque burden suggests a role of IL5 in men. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrews R.M., Shpitser I., Didelez V., Chaves P.H.M., Lopez O.L., Carlson M.C. Examining the causal mediating role of cardiovascular disease on the effect of subclinical cardiovascular disease on cognitive impairment via separable effects. J Gerontol. A Biol. Sci. Med. Sci. 2023;78:1172–1178. doi: 10.1093/gerona/glad077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien C.F., Huang L.C., Li K.Y., Yang Y.H. Cognitive effects of cilostazol in Alzheimer's dementia patients with peripheral arterial occlusive disease: A case-control study. Geriatr. Gerontol. Int. 2023;23:194–199. doi: 10.1111/ggi.14542. [DOI] [PubMed] [Google Scholar]
- 36.Murabito J.M., White C.C., Kavousi M., Sun Y.V., Feitosa M.F., Nambi V., Lamina C., Schillert A., Coassin S., Bis J.C., et al. Association between chromosome 9p21 variants and the ankle-brachial index identified by a meta-analysis of 21 genome-wide association studies. Circulation. Circ. Cardiovasc. Genet. 2012;5:100–112. doi: 10.1161/circgenetics.111.961292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullo I.J., Turner S.T., Kardia S.L.R., Mosley T.H., Jr., Boerwinkle E., de Andrade M. A genome-wide linkage scan for ankle-brachial index in African American and non-Hispanic white subjects participating in the GENOA study. Atherosclerosis. 2006;187:433–438. doi: 10.1016/j.atherosclerosis.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Freeman G., Cowling B.J., Schooling C.M. Power and sample size calculations for Mendelian randomization studies using one genetic instrument. Int. J. Epidemiol. 2013;42:1157–1163. doi: 10.1093/ije/dyt110. [DOI] [PubMed] [Google Scholar]
- 39.Xu S., Liu Y., Wang Q., Liu F., Xu F., Liu Y. Mendelian randomization study reveals a causal relationship between coronary artery disease and cognitive impairment. Front. Cardiovasc. Med. 2023;10 doi: 10.3389/fcvm.2023.1150432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tariq S., Barber P.A. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. 2018;144:565–581. doi: 10.1111/jnc.14132. [DOI] [PubMed] [Google Scholar]
- 41.Petrova M., Gavino A., Li Y., McLachlan C.S. Comparison of Parameters for Assessment of Carotid Stiffness and Their Association with Carotid Atherosclerosis in Rural Australian Adults: A Pilot Study. J. Clin. Med. 2023;12 doi: 10.3390/jcm12082935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cecelja M., Keehn L., Ye L., Spector T.D., Hughes A.D., Chowienczyk P. Genetic aetiology of blood pressure relates to aortic stiffness with bi-directional causality: evidence from heritability, blood pressure polymorphisms, and Mendelian randomization. Eur. Heart J. 2020;41:3314–3322. doi: 10.1093/eurheartj/ehaa238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baier D., Teren A., Wirkner K., Loeffler M., Scholz M. Parameters of pulse wave velocity: determinants and reference values assessed in the population-based study LIFE-Adult. Clin. Res. Cardiol. 2018;107:1050–1061. doi: 10.1007/s00392-018-1278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pewowaruk R., Korcarz C., De Boer I., Kestenbaum B., Heckbert S.R., Tedla Y.G., Gepner A.D. Carotid Artery Stiffness Mechanisms Are Associated With End Organ Damage and All-Cause Mortality: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Heart Assoc. 2023;12 doi: 10.1161/jaha.122.027517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Popele N.M., Grobbee D.E., Bots M.L., Asmar R., Topouchian J., Reneman R.S., Hoeks A.P., van der Kuip D.A., Hofman A., Witteman J.C. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 46.Sato H., Hayashi J., Harashima K., Shimazu H., Kitamoto K. A population-based study of arterial stiffness index in relation to cardiovascular risk factors. J. Atheroscler. Thromb. 2005;12:175–180. doi: 10.5551/jat.12.175. [DOI] [PubMed] [Google Scholar]
- 47.Allison E.Y., Al-Khazraji B.K. Association of Arterial Stiffness Index and Brain Structure in the UK Biobank: A 10-Year Retrospective Analysis. Aging Dis. 2023 doi: 10.14336/ad.2023.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecelja M., Jiang B., Bevan L., Frost M.L., Spector T.D., Chowienczyk P.J. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. J. Am. Coll. Cardiol. 2011;57:1480–1486. doi: 10.1016/j.jacc.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rode M., Teren A., Wirkner K., Horn K., Kirsten H., Löffler M., Scholz M., Pott J. Genome-wide association analysis of pulse wave velocity traits provide new insights into the causal relationship between arterial stiffness and blood pressure. PLoS One. 2020;15:e0237237. doi: 10.1371/journal.pone.0237237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skrivankova V.W., Richmond R.C., Woolf B.A.R., Yarmolinsky J., Davies N.M., Swanson S.A., VanderWeele T.J., Higgins J.P.T., Timpson N.J., Dimou N., et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614–1621. doi: 10.1001/jama.2021.18236. [DOI] [PubMed] [Google Scholar]
- 51.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savage J.E., Jansen P.R., Stringer S., Watanabe K., Bryois J., de Leeuw C.A., Nagel M., Awasthi S., Barr P.B., Coleman J.R.I., et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat. Genet. 2018;50:912–919. doi: 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry B.I., Upthegrove R., Kappelmann N., Jones P.B., Burgess S., Khandaker G.M. Associations of immunological proteins/traits with schizophrenia, major depression and bipolar disorder: A bi-directional two-sample mendelian randomization study. Brain Behav. Immun. 2021;97:176–185. doi: 10.1016/j.bbi.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierce B.L., Ahsan H., Vanderweele T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011;40:740–752. doi: 10.1093/ije/dyq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howrigan D.P., Rkwalters D.P., Francioli L., Hammerbacher J. Zenodo; 2023. Nealelab/UK_Biobank_GWAS: v2 (Version v2) [Google Scholar]
- 56.Ruth Mitchell E., B.L., Mitchell R., Raistrick C.A., Paternoster L., Hemani G., Gaunt T.R. BioRxiv; 2019. MRC IEU UK Biobank GWAS Pipeline Version 2. [Google Scholar]
- 57.Kurki M.I., Karjalainen J., Palta P., Sipilä T.P., Kristiansson K., Donner K.M., Reeve M.P., Laivuori H., Aavikko M., Kaunisto M.A., et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–518. doi: 10.1038/s41586-022-05473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Harst P., Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018;122:433–443. doi: 10.1161/circresaha.117.312086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Deerlin V.M., Sleiman P.M.A., Martinez-Lage M., Chen-Plotkin A., Wang L.S., Graff-Radford N.R., Dickson D.W., Rademakers R., Boeve B.F., Grossman M., et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat. Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chia R., Sabir M.S., Bandres-Ciga S., Saez-Atienzar S., Reynolds R.H., Gustavsson E., Walton R.L., Ahmed S., Viollet C., Ding J., et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet. 2021;53:294–303. doi: 10.1038/s41588-021-00785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burgess S., Butterworth A., Thompson S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bowden J., Del Greco M F., Minelli C., Davey Smith G., Sheehan N.A., Thompson J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 2016;45:1961–1974. doi: 10.1093/ije/dyw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartwig F.P., Davey Smith G., Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bowden J., Spiller W., Del Greco M F., Sheehan N., Thompson J., Minelli C., Davey Smith G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018;47:1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgess S., Thompson S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017;32:377–389. doi: 10.1007/s10654-017-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgess S., Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016;40:597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staley J.R., Blackshaw J., Kamat M.A., Ellis S., Surendran P., Sun B.B., Paul D.S., Freitag D., Burgess S., Danesh J., et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamat M.A., Blackshaw J.A., Young R., Surendran P., Burgess S., Danesh J., Butterworth A.S., Staley J.R. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35:4851–4853. doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larsson S.C., Traylor M., Malik R., Dichgans M., Burgess S., Markus H.S., CoSTREAM Consortium, on behalf of the International Genomics of Alzheimer’s Project Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ (Clinical research ed.) 2017;359:j5375. doi: 10.1136/bmj.j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yarmolinsky J., Relton C.L., Lophatananon A., Muir K., Menon U., Gentry-Maharaj A., Walther A., Zheng J., Fasching P., Zheng W., et al. Appraising the role of previously reported risk factors in epithelial ovarian cancer risk: A Mendelian randomization analysis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L., Tang L., Huang T., Fan D. Life Course Adiposity and Amyotrophic Lateral Sclerosis: A Mendelian Randomization Study. Ann. Neurol. 2020;87:434–441. doi: 10.1002/ana.25671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SNP, single-nucleotide polymorphism; CHR, chromosome; POS, position; OA, other_allele; EA, effect_allele; SE, standard error; CPAmax, area of the largest carotid plaque detected; CPSmax, maximal degree of stenosis; PWV, pulse wave velocity; baPWV, brachial-ankle PWV; bfPWV, brachial-femoral PWV; PAD, peripheral artery disease; pwASI, pulse wave arterial stiffness index; CoAS, coronary atherosclerosis; CeAS, cerebral atherosclerosis; ATHSCLE, atherosclerosis, excluding cerebral, coronary and PAD; CAD, coronary artery disease; AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; VaD, vascular dementia; MR, Mendelian randomization; F-statistic = Beta2/SE2; IVW, inverse variance weighted; MR-PRESSO, Mendelian randomization pleiotropy residual sum and outlier; OR, odd ratio; CI, confidence interval.
Data Availability Statement
This paper analyzes existing, publicly available data. These accession websites for the datasets are listed in the key resources table. The original code is available in this paper’s supplemental information (Methods S1). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.