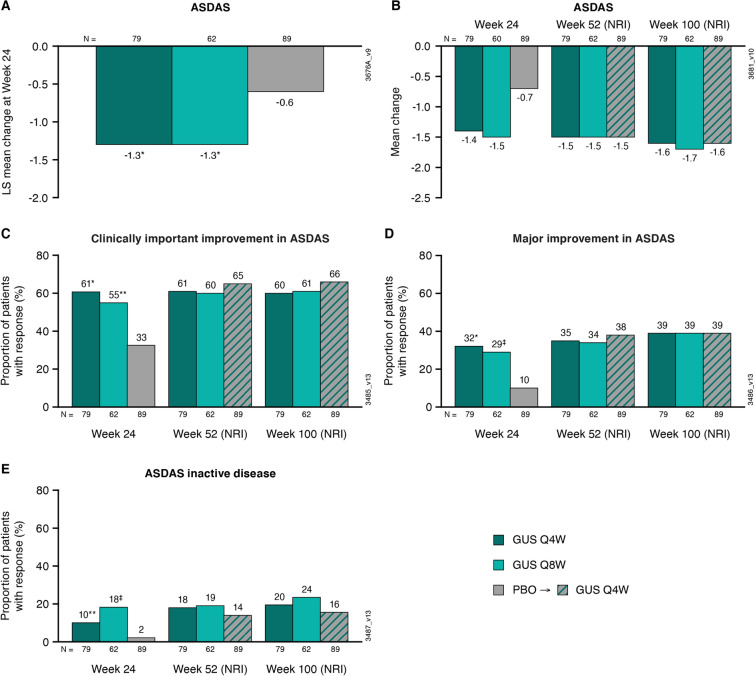
Fig. 2.
LS mean changes from baseline to week 24 in patients from DISCOVER-2 with active psoriatic arthritis and investigator-verified, imaging-confirmed sacroiliitis in ASDAS (A); mean changes from baseline to weeks 24, 52, and 100 in ASDAS (B), and the proportions of patients achieving a ASDAS clinically important improvement (decrease ≥ 1.1) (C), ASDAS major improvement (decrease ≥ 2.0) (D), and ASDAS inactive disease (< 1.3) (E) at weeks 24, 52, and 100. Through week 24, treatment failure rules were applied, and LS mean changes were determined utilizing MMRM. After week 24, patients with missing data were considered nonresponders or to have no change from baseline (nonresponder imputation; NRI). Treatment group comparisons (each guselkumab group vs. placebo at week 24) were performed for LS mean change in ASDAS and ASDAS response rates. ASDAS Ankylosing Spondylitis Disease Activity Score, GUS guselkumab, LS least squares, MMRM mixed-effect model for repeated measures, NRI nonresponder imputation, PBO placebo, Q4W/Q8W every 4/8 weeks. Unadjusted p value vs. placebo: *p < 0.001; ‡p ≤ 0.01; **p < 0.05