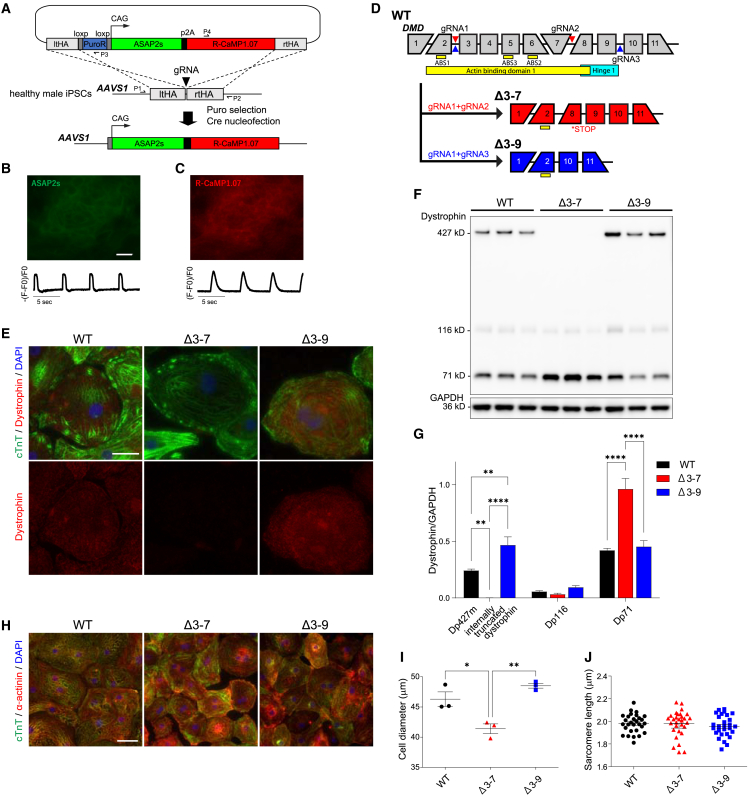
Figure 1.
Generation of isogenic cell lines with in-frame and out-of-frame deletions in the ABD1 coding region of DMD harboring genetically encoded dual indicator for voltage and calcium in AAVS1
(A) An expression cassette of genetically encoded action potential indicator ASAP2s (green) and calcium indicator R-CaMP1.07 (red) was engineered into intron 1 of AAVS1, a safe harbor in a healthy human male hiPSC by CRISPR-Cas9 editing. (B and C) Representative fluorescent images of spontaneously beating hiPSC-CMs (WT, on day 50) showing voltage (ASAP2s) (B, top) and intracellular calcium (R-CaMP1.07) (C, top), and representative traces of voltage (B, bottom) and intracellular calcium ions (C, bottom). Scale bar, 20 μm. (D) Generation of isogenic cell lines with the DMD Δ37 and Δ3–9 from WT hiPSCs carrying ASAP2s/RCaMP1.07 (A) by genome editing using gRNAs targeting deep introns. (E) Immunostaining of cTnT (green) and dystrophin (red) in WT, Δ3–7, and Δ3–9 hiPSC-CMs on day 48 after differentiation. DNA was counterstained with DAPI. Scale bar, 20 μm. (F) Western blot analysis showing protein levels of three isoforms of dystrophin protein, Dp427, internally truncated dystrophin, Dp116, and Dp71 in day 48 WT, Δ3–7, and Δ3–9 hiPSC-CMs. GAPDH was used as a loading control. (G) Quantification of dystrophin isoform protein in F (n = 3 independent CM differentiation batches per group). (H) Immunostaining of cTnT (green) and α-actinin (red) in day 48 control WT, Δ3–7, and Δ3–9 hiPSC-CMs. DNA was counterstained with DAPI. Scale bar, 50 μm. (I) Cell diameter determined by forward scatter in Flow cytometry using the cTnT antibody on WT, Δ3–7, and Δ3–9 hiPSC-CMs on day 48 after differentiation. (J) Sarcomere length of day 48 hiPSC-CMs. Mean length: WT, 1.981 μm; Δ3–9, 1.956 μm; and Δ3–7, 1.979 μm (n = 30). Data are presented as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001.