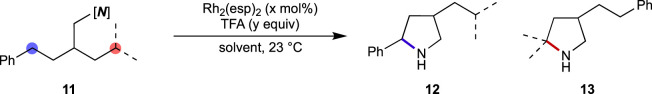
TABLE 2.
Comparison between O-Bz and O-Ts hydroxylamines using rhodium catalyst. a
|
| Entry | 11 | Conditions | Yield (%) | 12:13 | anti/syn (12) |
|---|---|---|---|---|---|
| 1 |

|
A | 73 | 31:69 | 83/17 |
| 2 |

|
B | 77 | 43:57 | 84/16 |
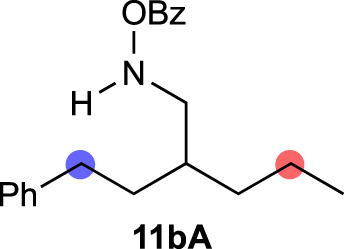
| 3 |
|
A | 83 | >96:4 | 81/19 |
| 4 |

|
B | 84 | >96:4 | 88/12 |
| 5 |

|
A | 82 | >96:4 | 83/17 |
| 6 |

|
B | 83 | >96:4 | 89/11 |
Conditions A: Rh2(esp)2 (1 mol%), TFA (10 equiv), HFIP., Conditions B: Rh2(esp)2 (2 mol%), TFA (2 equiv), TFE.
Products were isolated after conversion into the corresponding N-Ts adducts. TFE; 2,2,2-trifluoroethanol, Ts; p-toluenesulfonyl.
