Figure 1.
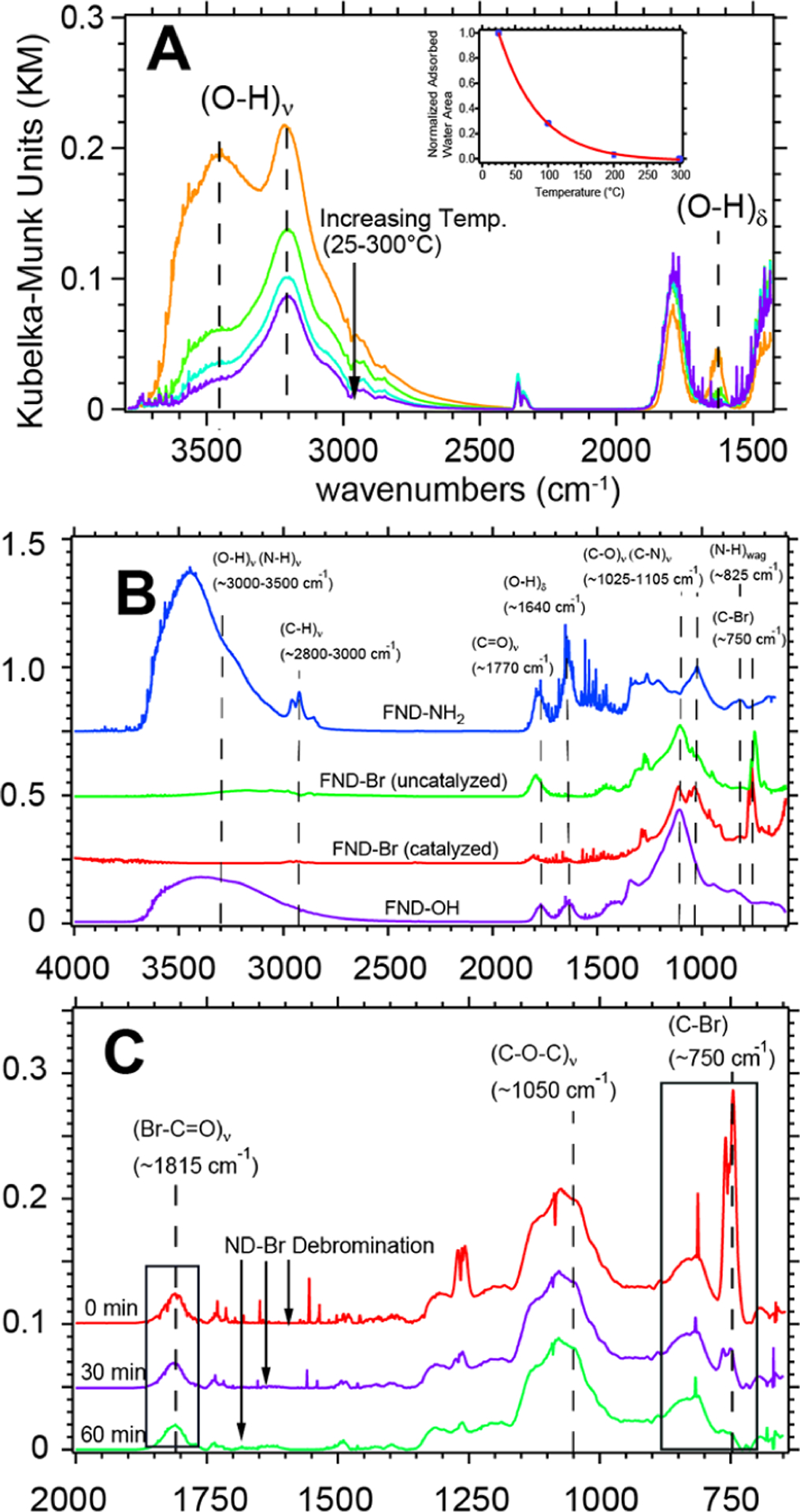
DRIFTS data showing the transition from ND–OH → ND–Br → ND–NH2 under open-air and inert atmosphere conditions. TPD-DRIFTS data from 25–300 °C confirm the contribution of (O–H)ν stretching modes due to both adsorbed water and the hydroxyl-terminated diamond (panel A). The (O–H)ν band from 3000–3500 cm−1 decreases proportionally to the elimination of (O–H)δ at 1630 cm−1. The inset shows the integrated (O–H)δ signal in Kubelka–Munk units as a function of temperature. DRIFTS spectra reveal a strong (C–Br)ν signal at 750 cm−1 that confirms alkyl bromide formation on the ND surface after addition of SOBr2 for 24 h with and without the presence of pyridine (panel B). After amination chemistry, ND–NH2 becomes highly hydrophilic; the (C–N)ν mode becomes prominent at 1025 cm−1, a small (N–H)wag signal at 825 cm−1 is observed, and the (C–Br)ν peak is absent (Panel B). In situ reactivity of the alkyl bromides under open-air conditions was tracked by opening two small valves on an inert atmosphere DRIFTS chamber and monitoring the decrease in the (C–Br)ν intensity at 750 cm−1 with pseudo-first-order kinetics (panel C). Note: During the debromination process, the (C–O–C)ν signal at 1075 cm−1 remained unchanged and did not convert readily back to the representative alcohol peak position of 1100 cm−1.
