Fig. 7 |. Mathematical modeling predicts that stromal cell-induced epithelial proliferation leads to increased lifetime breast cancer risk in BRCA1+/mut.
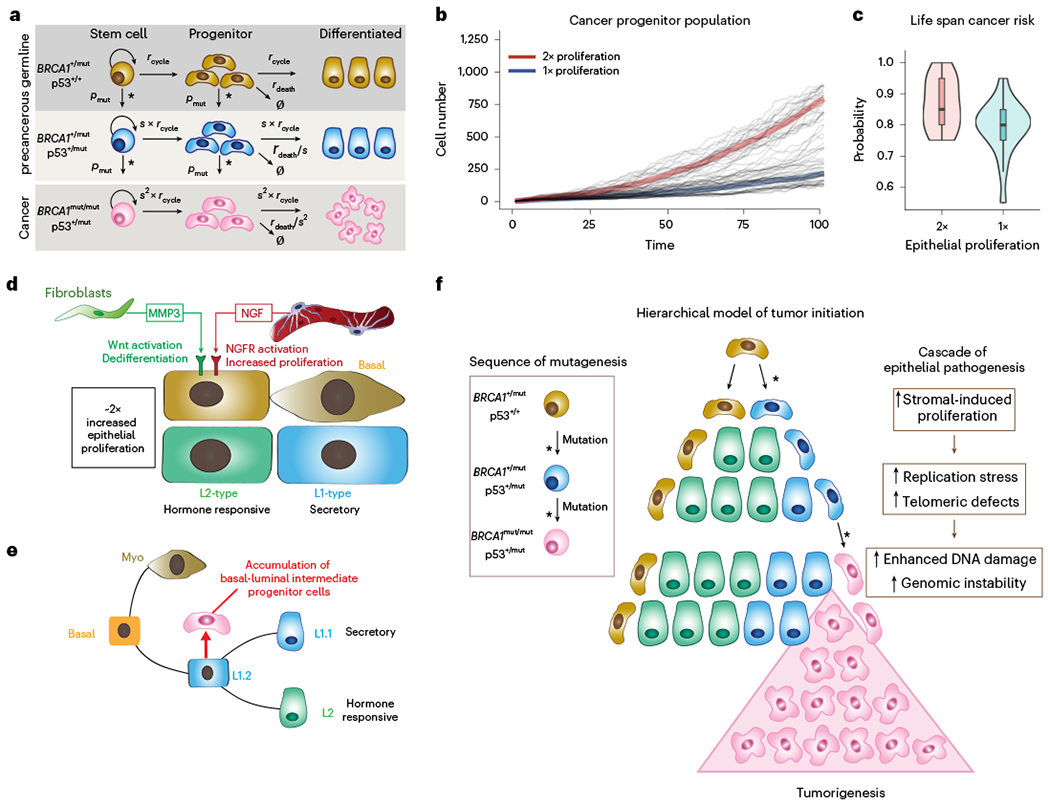
a, Schematic model illustrating the assumptions and parameters used to simulate the sequential mutations in oncogenes in BRCA1+/mut cells. rcycle is the baseline cell division rate, rdeath is the cell date rate, s is the proliferation scale factor and pmut is the probability of acquiring a variant in a driver oncogene. Parameters are further defined in Supplementary Table 17. b, Comparison between cancer progenitor population dynamics as predicted by a hierarchical model34. Thick lines: Average population dynamics of proliferation in a population with a twofold increase in proliferation and control group (blue). Gray thin lines: The stochastic simulation trajectories (sample n = 50 for each group). c, Comparison of predicted risk ratio of cancer initiation between twofold (red) and onefold epithelial proliferation rate (blue) over human lifespan. The samples are collected from the simulation of n = 40 patients in two groups, with the risk ratio of each patient estimated from n = 20 simulations of a random mutation model36. Violin plots show the distribution of risk ratios over n = 20 patients in each group, and boxplots indicate median and 25 and 75% quantiles, respectively; minima and maxima represent the 10th and 90th percentile, respectively. Wilcoxon test: P = 0.011. d, Schematic illustrating the concept of a pro-proliferative stromal niche in preneoplastic BRCA1+/mut breast tissues. BRCA1+/mut stromal cells express increased levels of pro-proliferative cues including NGF in pericytes and protumorigenic MMP3 in fibroblasts. e, We propose that stromal cues act in concert during the preneoplastic phase to promote the expansion of a subset of basal-luminal intermediate progenitor cells as potential cancer cells of origin. f, Concept illustration of hierarchical model of cancer initiation in BRCA1+/mut. Sequences of mutations are indicated in differently colored cells in box on the left; an asterisk represents a mutagenic event. Center schematic summarizes the outcome of mathematical modeling results, indicating expansion of cancer progenitors and ultimately leading to tumorigenesis. Cascade of epithelial cell-intrinsic events promoting tumorigenesis in BRCA1+/mut is shown on the right. Due to increased stromal cell-induced proliferation and replication stress, BRCA1+/mut breast epithelial stem cells accumulate mutations and become genomically instable, which ultimately drives tumor initiation.
