Abstract
Background
Current knowledge regarding coronavirus disease 2019 (COVID-19) is constantly evolving, and the long-term functional impairments, limitations, and restrictions have not yet been well established.
Objective
to evaluate the impact of post-COVID condition on the human functioning through the International Classification of Functioning, Disability and Health (ICF) classification.
Methods
This is a prospective cohort study with 53 individuals with post-COVID condition at 3 time points: 0 to 3 (baseline), 3 to 6, and 6-12 months (follow-up). Outcomes were organized in dichotomous variable: No impairment (0); presence of impairment (≥1) in body function, structure, activities, and participation domains according to the ICF checklist. Chi-square test was used to determine the differences of 3 time points, and association with persistent symptoms.
Results
A statistically significant difference was observed between the periods, with greater disabilities at 6-12 than at 0-3 months in mental, sensory, pain, and movement-related functions; cardiovascular, immunological, and respiratory systems. In terms of activity and participation, a greater limitation at 6-12 months was observed than at 0-3 months in learning and applying knowledge, general tasks, and mobility. In the domain of interpersonal interactions and relationships, there was a statistically significant difference between the 6-12 and 3-6 months groups. Associations between COVID-19 symptoms and ICF components at the first follow-up were: anosmia and dysgeusia with weight maintenance, fatigue and irritability with pain, brain fog with watching and listening, walking difficulty with pain, and headache with pain, watching, and listening. At the second follow-up were: anosmia and dysgeusia with energy and drive functions, attention, memory, and emotional functions; dizziness with watching and listening; fatigue with emotional function, pain, undertaking multiple tasks, lifting and carrying objects, and driving; irritability with energy and drive, emotional function, undertaking multiple tasks, lifting and carrying objects, and walking; walking difficulty with energy and driving, emotional function, respiration, muscle power, cardiovascular system, undertaking multiple tasks, lifting and carrying objects, and walking; and headache with emotional function, watching, and listening.
Conclusions
Individuals with COVID-19 persistent symptoms showed impairments in structure and function, activity limitations, and participation restrictions during the 1-year follow-up period.
Keywords: COVID-19, body function, activity limitations, participation restrictions, International classification of functioning, disability and health
Introduction
The coronavirus disease 2019 (COVID-19) can have long-term consequences after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. 1 Moreover, the current literature suggests that SARS-CoV-2 is not confined to the respiratory tract, but systemic involvement seems to occur in these patients. 2 Havervall et al showed that even individuals with mild-to-moderate COVID-19 reported a variety of long-term symptoms and that these symptoms disrupted work, social life, and personal life. 3
Functional impairment in patients after the acute phase of COVID-19 has not been well documented, and the only available information is provided by expert opinions. 4 The complexity and variability of the long-term clinical and functional manifestations of COVID-19 cannot be supported by a biomedical model alone, 2 but should be explored in a biopsychosocial context. However, COVID-19 might have detrimental sequelae even after the post-acute phase, depicting a new pathological condition: the “post-COVID-19 syndrome (PCS), long COVID, 5 or post-COVID condition is suggested by the World Health Organization. 6 As it is a relatively new disease with multisystemic repercussions, studies are needed to classify functioning, disability, and health to better understand long-term impairments and perform more specific and effective interventions to reduce the functional impact on activity and participation.
To describe human functioning, the World Health Organization (WHO) developed the International Classification of Functioning, Disability, and Health (ICF) to understand the clinical and functional aspects of individuals through a standard language. 7 Although appropriate for all populations, it has been applied mainly in the neurological, musculoskeletal, and work-related contexts. 8 The ICF classification enables to view the individual from a systemic point of view using a biopsychosocial model; however, a few studies exist looking at functioning in post-COVID condition9,10 but not using the ICF.
Current knowledge on COVID-19 is constantly evolving, and the long-term clinical and functional aspects after acute infection have not yet been well established in the literature. Impairments in structure and body function are well documented;11-14 however, the functional range and impact on activity and participation require further investigation. Andrenelli et al. highlighted that studies with high levels of evidence regarding long-term monitoring remain lacking. 15 Many studies have already explored changes in structures and body functions, and the use of the ICF can provide rich data on the biopsychosocial aspects, including activity limitations and participation restrictions, of the post-COVID condition. Therefore, this study aimed to evaluate the impact of COVID on human functioning through the ICF classification.
Methods
Study design, setting and participants
This 12-month prospective cohort study included COVID-19 patients. This study was performed at the Neuroscience and Motor Control Laboratory between September 2020 and September 2021. COVID-19 diagnosis was confirmed using SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) of nasopharyngeal swab samples. Individuals were recruited via radio, television, and digital media and referred to the Uberaba Municipal Health Department. This study was approved by the Research Ethics Committee of the Federal University of Triângulo Mineiro (UFTM) (CAAE: 30684820.4.0000.5154). Participants signed an informed consent form to participate in the study.
Inclusion criteria
1) Individuals with mild-to-moderate COVID-19 persistent symptoms who met the post-COVID condition. Post-COVID condition (long COVID) was defined as the continuation or development of new symptoms consistent with COVID-19 infection that continues for more than 12 weeks and is not explained by an alternative diagnosis. 16 The diagnosis of long COVID was based on medical evaluation, current symptoms, and complementary examinations;
2) Older adults (age between 18 to 65 years);
3) No previous disabilities (modified Rankin Scale score <1); 17
4) Have more than 9 years of schooling (completed at least high school) because cognitive activity may vary according to educational level and could interfere with the ICF analysis. In Latin America, low educational levels are associated with a higher risk of dementia. 18
Exclusion criteria
Exclusion criteria were adopted because they could increase baseline disability and act as confounders.
1) Patients with severe and critical COVID-19;
2) Previous history of mental and cognitive disorders;
3) If they did not attend reassessments during follow-up;
4) Have a neurological or psychiatric disease unrelated to COVID-19 infection during follow-up (could be another confounder).
Variables
(1) Dichotomic outcomes: no impairment (grade 0); presence of impairment (grade ≥1) in the body function, structure, activities, and participation domains according to the ICF checklist classification.
(2) Other variables included age, sex, scholarity (years of study), employment, and initial symptoms.
Data sources and measurement
Patients with post-COVID condition were referred for triage. After inclusion in the study, the patients were interviewed and their personal and demographic data were recorded. All patients were followed up for 1 year at 3 moments: 0-3, 3-6, and 6-12 months. The ICF checklist7,19,20 was then applied through face-to-face interviews with the study participants by a trained and certified researcher. The components evaluated were body function (b)—physiological functions of body systems (including psychological functions), structure of the body (s)—anatomical parts of the body, such as organs, limbs, and their components, and activity and participation (d)— the execution of a task or action by an individual, and involvement in a life situation (Supplemental file 1). While 1 study demonstrated a set of categories identified for the acute, post-acute, and long-term, 21 it was not an official core set developed by the WHO Health Organization for post-COVID condition. 22 Therefore, we used the ICF checklist (Supplementary File 1).
The presence or absence of impairments was rated for the body function and structure domains (0, none; 1, mild; 2, moderate; 3, severe; and 4, complete). In the activity and participation domains, the presence or absence of limitations or restrictions was determined using the same score as above. For the analysis and classification of ICF, participants were followed up at 3 time points: 0-3, 3-6, and 6-12 months. The original scale was categorized dichotomously, and during each period, the participants were divided into 2 groups: absence of impairment/activity limitations/participation restrictions (0) and presence of impairment/activity limitations/participation restrictions (≥1).
Bias
The effort to address attrition bias involved sending routine reminders to schedule follow-ups. In addition, data on all potential confounders (age, sex, scholarity, employment, and initial symptoms) were collected.
Statistical methods
Data normality was measured using the Shapiro–Wilk test. Clinical and demographic data were measured using descriptive statistics as mean and standard deviation for continuous data and frequency and percentage for categorical data. We performed an ANOVA for continuous data and chi-square test for categorical data. The chi-square test was used to determine the differences in the matched sets of 3 frequencies (0-3 vs 3-6 vs 6-12 months). Statistical significance was set at P < .05. ICF items with statistical significance at 2 timepoints (3-6 and 6-12 months) were associated with persistent symptoms according to the chi-square test for trends. Statistical analysis was performed using GraphPad Prism version 8.0.0 for Macintosh (GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
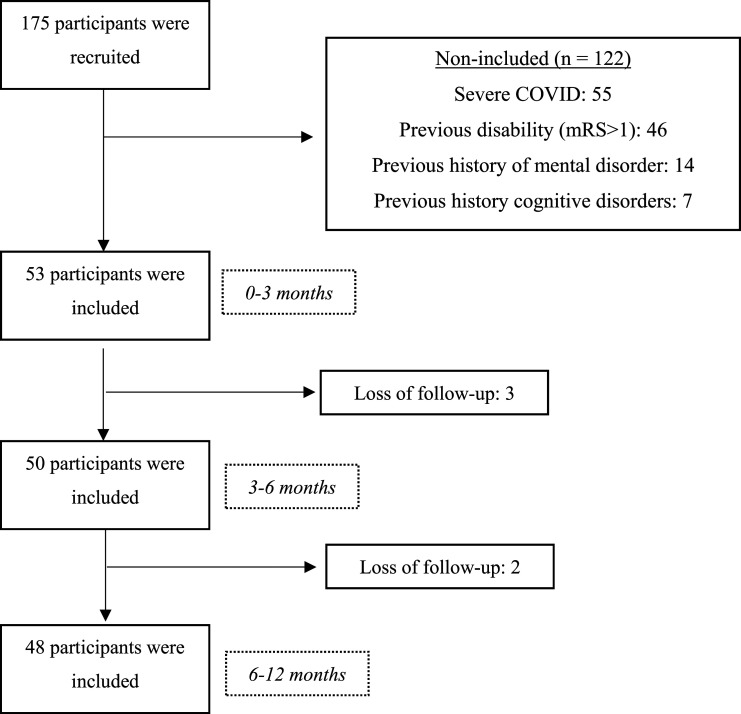
Fifty-three post-COVID-19 individuals participated in the study: 37 women (69.8%) and 16 men (30.2%) with a mean age of 41.4 ± 6.6 years. At the first follow-up (3-6 months), a loss of 5.7% of the participants (n = 50; 35 women, 15 men, mean age: 40.3 ± 5.8 years) was noted. At the second follow-up (6-12 months), a total loss of 9.4% (n = 48, 34 women, 14 men, mean age: 40.7 ± 6.2 years) was noted (Figure 1). Three patients dropped out of the study between 0-3 and 3-6 months, and 2 dropped out between 3-6 and 6-12 months. Among all the dropouts, a lack of interest was noted in participating in the study and failure to attend the scheduled evaluations. Participants who withdrew from the study during the follow-up did not differ from other participants in terms of sociodemographic data or disability. Sociodemographic data are presented in Table 1. No statistical differences were noted between the follow-up periods. Table 2 shows the symptoms that persisted, resolved, and recurred during the 1-year follow-up period. The increase in brain fog persisted at 6-12 months compared with baseline. Dizziness and irritability resolved to a greater proportion at 3-6 months compared with at 6-12 months.
Figure 1.
Study flowchart.
Table 1.
Demographic, clinical, and personal data during 1 year of follow-up.
| Evaluation time | ||||
|---|---|---|---|---|
| Variables | 0-3 months (n = 53) | 3-6 months (n = 50) | 6-12 months (n = 48) | P-value |
| Mean±SD | Mean±SD | Mean±SD | ||
| Age (years)1 | 41.4±6.6 | 40.3±5.8 | 40.7±6.2 | .878 |
| Scholarity (years)1 | 14.4±3.4 | 14.0±3.0 | 14.2±3.1 | .934 |
| Sex2 | ||||
| Female, n (%) | 37 (69.8) | 35 (70.0) | 34 (70.8) | .677 |
| Male, n (%) | 16 (30.2) | 15 (30.0) | 14 (29.2) | .677 |
| Employed, n (%)2 | 50 (94.3) | 47 (94.0) | 45 (93.7) | .941 |
| Student, n (%)2 | 2 (3.8) | 2 (4.0) | 2 (4.2) | .952 |
| Retired, n (%)2 | 1 (1.9) | 1 (2.0) | 1 (2.1) | >.99 |
| Drop out patients | 0 (.0) | 3 (6.0) | 2 (4.2) | .236 |
| Lack of interest | 0 (.0) | 1 (2.0) | 1 (2.1) | .584 |
| Failure to attend | 0 (.0) | 2 (4.0) | 1 (2.1) | .361 |
| Age (years)3 | - | 42.1±4.1 | 41.8±5.2 | .678 |
| Scholarity (years)3 | - | 14.5±2.5 | 14.0±3.0 | .899 |
| Sex2 | ||||
| Female, n (%) | - | 2 (66.7) | 1 (50.0) | >.99 |
| Male, n (%) | - | 1 (33.3) | 1 (50.0) | >.99 |
| Employed, n (%)2 | - | 3 (100.0) | 2 (100.0) | >.99 |
SD: standard deviation; 1 – ANOVA test; 2 – Chi-square test; 3 – Mann–Whitney U test (comparing dropout patients with non-dropout patients).
Table 2.
Symptoms that persist, resolve, and reoccur during 1 year of follow-up.
| 0-3 months (n = 53) | 3-6 months (n = 50) | 6-12 months (n = 48) | |||||
|---|---|---|---|---|---|---|---|
| Symptoms | Baseline | Persist a | Resolve | Recurred | Persist a | Resolve | Recurred |
| Anosmia, n (%)2 | 32 (60.4) | 24 (48.0) | 8 (16.0) | 0 (0) | 22 (45.8) | 2 (4.2) | 0 (.0) |
| Dysgeusia, n (%)2 | 28 (52.8) | 24 (48.0) | 4 (8.0) | 0 (0) | 20 (41.6) | 4 (8.3) | 0 (.0) |
| Dizziness, n (%)2 | 16 (30.2) | 9 (18.0) | 7 (14.0) a | 0 (0) | 10 (20.8) | 0 (.0) a | 1 (2.1) |
| Fatigue, n (%)2 | 15 (28.3) | 16 (32.0) | 0 (.0) | 1 (2.0) | 15 (31.2) | 1 (2.1) | 0 (.0) |
| Irritability, n (%)2 | 10 (18.9) | 15 (30.0) | 5 (10.0) a | 0 (.0) | 16 (33.3) | 0 (.0) a | 1 (2.1) |
| Brain fog, n (%)2 | 9 (17.0) | 14 (28.0) | 0 (.0) | 5 (10.0) | 16 (33.3) b | 0 (.0) | 2 (4.2) |
| Walking difficulty, n (%)2 | 9 (17.0) | 12 (24.0) | 0 (.0) | 3 (6.0) | 13 (27.1) | 0 (.0) | 1 (2.1) |
| Headache, n (%)2 | 8 (15.1) | 13 (26.00 | 0 (.0) | 5 (10.0) a | 12 (25.0) | 1 (2.1) | 0 (.0)a |
aSymptoms that persisted were from the same patient as those in the previous evaluation.
bBaseline comparison (P < .05).
Same letters indicate significant differences between the periods.
The structure and body function impairments, activity limitations, and participation restrictions in patients with COVID-19 for over 1 year are shown in Table 3. A statistically significant difference was between the periods, with greater impairments at 6-12 than at 0-3 months in mental functions (P = .005); sensory functions and pain (P = .023); neuromusculoskeletal and movement-related functions (P = .003); and cardiovascular, immunological, and respiratory systems (P = .002). In terms of activity and participation, there was a statistically significant difference between the periods, with greater limitations/restrictions at 6-12 months than at 0-3 months in terms of learning and applying knowledge (P = .005), general tasks and demands (P = .006), and mobility (P = .001). In the domain of interpersonal interactions and relationships, a statistically significant difference was noted between the 6-12 and 3-6 months groups (P = .02) (Table 3).
Table 3.
Structure and body function impairments, activity limitations, and participation restrictions in patients with COVID-19 over 1 year.
| 0-3 (n = 53) | 3-6 (n = 50) | 6-12 (n = 48) | p | |
|---|---|---|---|---|
| Structure and body function (b,s) | ||||
| b1. Mental functions (n, %) | 9 (16.9)a,c | 11 (22.0) b | 13 (27.1)c,a | .005 |
| b2. Sensory functions and pain (n, %) | 10 (18.9)a,c | 11 (22.0) b | 15 (31.2)c,a | .023 |
| b3. Voice and speech functions (n, %) | 0 (0) a | 5 (10.0) b | 5 (10.4) c | .321 |
| b4. Functions of the cardiovascular, hematological, immunological and respiratory systems (n, %) | 5 (9.4) a | 5 (10.0) b | 5 (10.4) c | .788 |
| b5. Functions of the digestive, metabolic, and endocrine systems (n, %) | 3 (5,7) a | 2 (4.0) b | 2 (4.2) c | .589 |
| b6. Genitourinary and reproductive functions (n, %) | 1 (1,8) a | 1 (2.0) b | 1 (2.1) c | .763 |
| b7. Neuromusculoskeletal and movement-related functions (n, %) | 8 (15,1)a,c | 11 (22.0) b | 16 (33.3)c,a | .003 |
| s1. Structure of the nervous system (n, %) | 1 (1.8) a | 1 (2.0) b | 0 (.0) c | .442 |
| s2. The eye, ear, and related structures (n, %) | 0 (.0) a | 1 (2.0) b | 0 (.0) c | .889 |
| s4. Structure of the cardiovascular, immunological, and respiratory systems (n, %) | 0 (.0)a,c | 3 (6.0) b | 8 (16.7)c,a | .002 |
| s5. Structures related to the digestive, metabolism, and endocrine systems (n, %) | 0 (.0) a | 0 (.0) b | 1 (2.1) c | .690 |
| s7. Structure related to movement (n, %) | 5 (9.4) a | 5 (10.0) b | 4 (8.3) c | .722 |
| Activity and participation (d) | ||||
| d1. Learning and applying knowledge (n, %) | 7 (13.2)a,c | 12 (24.0) b | 17 (35.4)c,a | .005 |
| d2. General tasks and demands (n, %) | 4 (7.5)a,c | 8 (16.0) b | 12 (25.0)c,a | .006 |
| d3. Communication (n, %) | 5 (9.4) a | 8 (16.0) b | 10 (20.8) c | .688 |
| d4. Mobility (n, %) | 11 (20.7)a,c | 12 (24.0) b | 19 (39.5)c,a | .001 |
| d5. Self care (n, %) | 4 (7.5) a | 5 (10.0) b | 6 (12.5) c | .289 |
| d6. Domestic life (n, %) | 2 (3.8) a | 5 (10.0) b | 10 (20.8) c | .188 |
| d7. Interpersonal interactions and relationships (n, %) | 7 (13.2) a | 4 (8.0)b,c | 12 (25.0)c,b | .021 |
| d8. Major life areas (n, %) | 2 (3,8) a | 0 (0) b | 0 (0) c | .644 |
| d9. Community, social and civic life (n, %) | 4 (7.5) | 0 (0) b | 0 (0) c | .791 |
aIndicate significant differences between periods.
bIndicate significant differences between periods.
cIndicate significant differences between periods.
b: body function; s: structure; d: domain.
Detailed items of each ICF domain in patients with COVID-19 over 1 year are shown in Table 4. Within the structure and body function domain, the detailed items that obtained a statistically significant difference between the periods, with greater impairments at 6-12 months compared with the period 0-3 months, were energy and drive functions (P = .002), attention (P = .033), memory (P = .038), emotional functions (P = .012), seeing (P = .022), pain (P = .028), respiration (P = .021), weight maintenance (P = .031), muscle power (P = .042), muscle tone (P = .021), and the cardiovascular system (P = .021). In the activity and participation domain, the detailed items that showed a statistically significant difference between the periods, with greater limitations/restrictions at 6-12 months compared with the period 0-3 months, were watching (P = .003), listening (P = .003), undertaking multiple tasks (P = .028), lifting and carrying objects (P = .033), fine hand use (P = .021), walking (P = .033), and driving (P = .033) (Table 4).
Table 4.
Detailed items of each ICF domain in patients with COVID-19 over 1 year.
| Item | 0-3 (n = 53) | 3-6 (n = 50) | 6-12 (n = 48) | p |
|---|---|---|---|---|
| b110. Consciousness (n, %) | 0 (.0) a | 0 (.0) b | 0 (.0) c | .999 |
| b114. Orientation (n, %) | 0 (.0) a | 7 (14.0) b | 4 (8.3) c | .882 |
| b117. Intellectual (n, %) | 0 (.0) a | 1 (2.0) b | 0 (.0) c | .901 |
| b130. Energy and drive functions (n, %) | 1 (1.8)a,c | 5 (10.0) b | 16 (33.3)c,a | .002 |
| b134. Sleep (n, %) | 16 (30.2) a | 15 (30.0) b | 17 (35.4) c | .245 |
| b140. Attention (n, %) | 16 (30.8)a,b | 17 (34.0) b | 23 (47.9)c,a | .033 |
| b144. Memory (n, %) | 17 (32.1)a,c | 18 (36.0) b | 28 (58.3)c,a | .038 |
| b152. Emotional functions (n, %) | 7 (13.2)a,c | 12 (24.0) b | 16 (33.3)c,a | .012 |
| b156. Perceptual functions (n, %) | 1 (1.8) a | 5 (10.0) b | 4 (8.3) c | .609 |
| b164. Higher level cognitive functions (n, %) | 4 (7.5) a | 5 (10.0) b | 4 (8.3) c | .876 |
| b167. Language (n, %) | 4 (7.5) a | 5 (10.0) b | 4 (8.3) c | .322 |
| b210. Seeing (n, %) | 7 (13.2)a,c | 8 (16.0) b | 16 (33,3)c,a | .022 |
| b230. Hearing (n, %) | 4 (7.5) a | 5 (10.0) b | 4 (8.3) c | .441 |
| b235. Vestibular (n, %) | 4 (7.5) a | 5 (10.0) b | 8 (16.7) c | .552 |
| b280. Pain (n, %) | 10 (18.9)a,c | 18 (36.0)b,a | 25 (52.1)c,a | .028 |
| b310. Voice (n, %) | 0 (.0) a | 5 (10.0) b | 0 (.0) c | .992 |
| b410. Heart functions (n, %) | 0 (0) a | 5 (10.0) b | 5 (10.4) c | .889 |
| b420. Blood pressure (n, %) | 4 (7.5) a | 6 (16.0) b | 12 (25.0) c | .732 |
| b435. Immunological (n, %) | 0 (.0) a | 0 (.0) b | 2 (4.2) c | .223 |
| b440. Respiration (n, %) | 0 (.0)a,c | 5 (10.0)b,c | 7 (14.5)c,a | .021 |
| b515. Digestive (n, %) | 7 (13.2) a | 6 (16.0) b | 2 (4.2) c | .408 |
| b525. Defecation (n, %) | 7 (13.2) a | 6 (16.0) b | 2 (4.2) c | .408 |
| b530. Weight maintenance (n, %) | 1 (1.8)a,b | 16 (32.0)b,a | 5 (10.4) c | .031 |
| b555. Endocrine glands (n, %) | 1 (1.8) a | 0 (.0) b | 2 (4.2) c | .942 |
| b640. Sexual functions (n, %) | 0 (.0) a | 1 (2.0) b | 0 (.0) c | .992 |
| b620. Urination functions (n, %) | 1 (1.8) a | 0 (.0) b | 2 (4.2) c | .889 |
| b710. Mobility of joint (n, %) | 7 (13.2) a | 12 (24.0) b | 12 (25.0) c | .791 |
| b730. Muscle power (n, %) | 7 (13.2)a,c | 12 (24.0) b | 18 (37.5)c,a | .042 |
| b735. Muscle tone (n, %) | 0 (.0)a,c | 5 (10.0) b | 12 (25.0)c,a | .021 |
| b765. Involuntary movements (n, %) | 1 (1.8) a | 3 (6.0) b | 0 (.0) c | .866 |
| s110. Brain (n, %) | 0 (.0) a | 3 (6.0) b | 0 (.0) c | .887 |
| s120. Spinal cord and peripheral nerves (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .945 |
| s410. Cardiovascular system (n, %) | 0 (.0)a,c | 5 (10.0) b | 7 (14.5)c,a | .021 |
| s430. Respiratory system (n, %) | 0 (.0) a | 3 (6.0) b | 0 (.0) c | .992 |
| s710. Head and neck region (n, %) | 1 (1.8) a | 3 (6.0) b | 0 (.0) c | .866 |
| s720. Shoulder region (n, %) | 1 (1.8) a | 3 (6.0) b | 0 (.0) c | .866 |
| s730. Upper extremity (n, %) | 0 (.0) a | 0 (.0) b | 2 (4.2) c | .932 |
| s740. Pelvis (n, %) | 0 (.0) a | 0 (.0) b | 2 (4.2) c | .932 |
| s750. Lower extremity (n, %) | 0 (.0) a | 0 (.0) b | 2 (4.2) c | .932 |
| s760. Trunk (n, %) | 0 (.0) a | 0 (.0) b | 2 (4.2) c | .932 |
| d110. Watching (n, %) | 0 (.0)a,b,c | 16 (32.0)b,a | 16 (33,3)c,a | .003 |
| d115. Listening (n, %) | 0 (0)a,b,c | 16 (32.0)b,a | 16 (33,3)c,a | .003 |
| d140. Learning to read (n, %) | 7 (13.2) a | 11 (22.0) b | 16 (33.3) c | .177 |
| d145. Learning to write (n, %) | 7 (13.2) a | 11 (22.0) b | 16 (33.3) c | .177 |
| d150. Learning to calculate (n, %) | 7 (13.2) a | 11 (22.0) b | 16 (33.3) c | .177 |
| d175. Solving problems (n, %) | 7 (13.2) a | 11 (22.0) b | 16 (33.3) c | .177 |
| d210. Undertaking a single task (n, %) | 7 (13.2) a | 11 (22.0) b | 16 (33.3) c | .177 |
| d220. Undertaking multiple tasks (n, %) | 7 (13.2)a,c | 8 (16.0) b | 18 (37.5)c,a | .028 |
| d310. Communicating with -- receiving -- spoken messages (n, %) | 7 (13.2) a | 11 (22.0) b | 10 (20.8) c | .554 |
| d315. Communicating with -- receiving -- non-verbal messages (n, %) | 7 (13.2) a | 11 (22.0) b | 10 (20.8) c | .554 |
| d330. Speaking (n, %) | 4 (7.5) a | 12 (24.0) b | 10 (20.8) c | .807 |
| d335. Producing non-verbal messages (n, %) | 4 (7.5) a | 12 (24.0) b | 10 (20.8) c | .807 |
| d350. Conversation (n, %) | 4 (7.5) a | 12 (24.0) b | 10 (20.8) c | .807 |
| d430. Lifting and carrying objects (n, %) | 12 (22.6) a | 11 (22.0) b | 18 (37.5) c | .033 |
| d440. Fine hand use (n, %) | 10 (18.8)a,c | 11 (22.0) b | 19 (39.5)c,a | .021 |
| d450. Walking (n, %) | 12 (22.6) a | 11 (22.0) b | 18 (37.5) c | .033 |
| d465. Moving around using equipment (n, %) | 4 (7.5) a | 12 (24.0) b | 10 (20.8) c | .807 |
| d470. Using transportation (n, %) | 10 (18.8)a,c | 11 (22.0) b | 19 (39.5)c,a | .021 |
| d475. Driving (n, %) | 12 (22.6) a | 11 (22.0) b | 18 (37.5) c | .033 |
| d510. Washing oneself (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d520. Caring for body parts (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d530. Toileting (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d540. Dressing (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d550. Eating (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d560. Drinking (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d570. Looking after one's health (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d620. Acquisition of goods and services (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d630. Preparation of meals (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d640. Doing housework (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d660. Assisting others (n, %) | 4 (7.5) a | 5 (10.0) b | 7 (14.5) c | .632 |
| d710. Basic interpersonal interactions (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d720. Complex interpersonal interactions (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d730. Relating with strangers (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d740. Formal relationships (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d750. Informal social relationships (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d760. Family relationships (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d770. Intimate relationships (n, %) | 6 (11.3) a | 7 (14.0) b | 7 (14.5) c | .822 |
| d810. Informal education (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d820. School education (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d830. Higher education (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d850. Remunerative employment (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d860. Basic economic transactions (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d870. Economic self-sufficiency (n, %) | 1 (1.8) a | 0 (.0) b | 0 (.0) c | .903 |
| d910. Community life (n, %) | 4 (7.5) a | 0 (.0) b | 0 (.0) c | .833 |
| d920. Recreation and leisure (n, %) | 4 (7.5) a | 0 (.0) b | 0 (.0) c | .833 |
| d930. Religion and spirituality (n, %) | 4 (7.5) a | 0 (.0) b | 0 (.0) c | .833 |
| d940. Human rights (n, %) | 4 (7.5) a | 0 (.0) b | 0 (.0) c | .833 |
| d950. Political life and citizenship (n, %) | 4 (7.5) a | 0 (.0) b | 0 (.0) c | .833 |
aIndicate significant differences between periods.
bIndicate significant differences between periods.
cIndicate significant differences between periods.
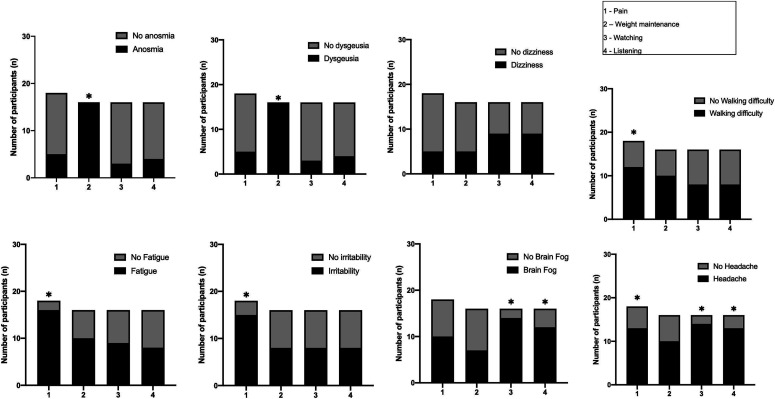
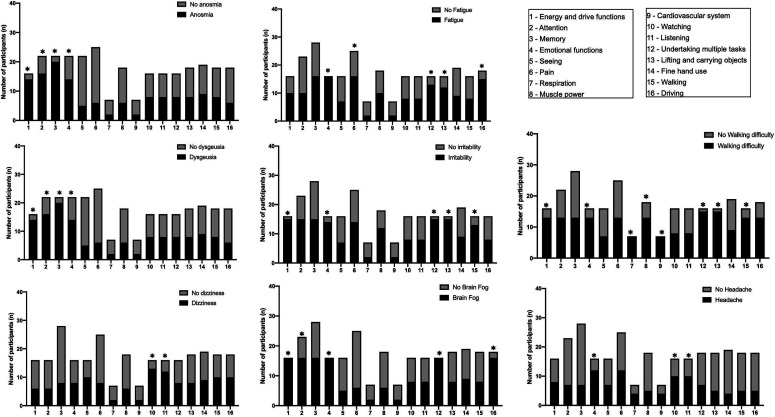
Figure 2 shows the associations between symptoms and ICF components at the first follow-up (3-6 months): Anosmia (P < .001) and dysgeusia (P < .001) with weight maintenance, fatigue (P = .002), and irritability (P = .024) with pain, brain fog with watching (P = .01) and listening (P = .021), walking difficulty with pain (P = .034), and headache with pain (P = .014), watching (P = .038), and listening (P = .038). Figure 3 shows the associations between symptoms and ICF components at the second follow-up (6-12 months): Anosmia with energy and drive functions (P = .002), attention (P = .031), memory (P = .003), and emotional functions (P = .042); dysgeusia with energy and drive functions (P = .001), attention (P = .034), memory (P = .008), and emotional function (P = .044); dizziness with watching (P = .008) and listening (P = .009); fatigue with emotional function (P < .001), pain (P = .003), undertaking multiple tasks (P = .022), lifting and carrying objects (P = .031), and driving (P = .014); irritability with energy and drive (P = .001), emotional function (P = .012), undertaking multiple tasks (P = .0018), lifting and carrying objects (P = .003), and walking (P = .021); brain fog with energy and drive (P < .001), attention (P = .026), emotional function (P < .001), undertaking multiple tasks (P < .001), and driving (P = .001); walking difficulty with energy and driving (P = .002), emotional function (P = .011), respiration (P < .001), muscle power (P = .002), cardiovascular system (P < .001), undertaking multiple tasks (P < .001), lifting and carrying objects (P < .001), and walking (P < .001); and headache with emotional function (P = .014), watching (P = .038), and listening (P = .038).
Figure 2.
Associations between symptoms and ICF components at first follow-up (3-6 months).
Figure 3.
Associations between symptoms and ICF components at second follow-up (6-12 months).
Discussion
Our study identified the most frequent changes, classified using the ICF, in post-COVID individuals over a period of 1 year. The main functional changes in the domains of body function and structure over a year were energy and drive functions; attention; memory; emotional functions; seeing, pain; respiration; weight maintenance; and muscle power, muscle tone, cardiovascular system. In the domains of activities and participation, the most reported limitations or restrictions over a year were watching, listening, undertaking multiple tasks, lifting and carrying objects, fine hand use, walking, and driving (Supplementary File 2).
Structure and body function impairments
Energy and drive functions are frequently impaired in post-COVID individuals from 6 to 12 months and are classified as general mental functions of physiological and psychological mechanisms that cause the individual to move toward satisfying specific needs and general goals in a persistent manner. 6 The main symptoms associated with these ICF components are anosmia, dysgeusia, irritability, brain fog, and difficulty in walking. A significant proportion of individuals experience these symptoms following resolution of acute COVID-19, due to direct viral encephalitis, neuroinflammation, 23 neurodegeneration, 24 cerebral microvascular injury, 1 and hypometabolism in areas associated with motivation, such as the dorsolateral prefrontal cortex 25 in the brains of COVID-19 patients.
Impairments related to attention and memory were observed at all time periods, with a higher frequency from 6 to 12 months, mainly in individuals with anosmia, dysgeusia, brain fog, or difficulty walking. In a meta-analysis, the prevalence of neurological symptoms was higher after 6 months, and the most frequent symptom was memory deficit. 26 Inflammation and oxidative stress during COVID-19 lead to secondary neuropathological dysfunction in addition to important cognitive impairments.24-26 Additionally, individuals with COVID-19 require social isolation, and consequences such as hysteria and loss of loved ones are associated with anxiety and depression. 27
Ocular manifestations (seeing) were more common at 3-6 months, with a higher proportion during 6 to 12 months after acute infection. Although none of the symptoms in the patients included in this study were associated with ocular manifestations, these impairments can be explained by vascular disorders and a high possibility of infection-induced clotting.28,29 The novel coronavirus SARS-CoV-2 has severe implications for ophthalmology as the eyes represent an important route of infection, most probably through lacrimal drainage into the nasal mucosa or ocular manifestations, which, even if rather rare, can represent the first symptoms of this novel disease.30,31
Pain was a frequent symptom that was more common during 3 to 6 months, with a higher proportion occurring during 6 to 12 months, mainly in individuals with fatigue and irritability. Bittencourt et al. highlighted the possibility that some patients develop pain symptoms post-COVID-19 due to post-viral syndrome or deterioration due to exacerbation of pre-existing physical symptoms, mental complaints, or lifestyle factors (eg, insomnia, physical inactivity). 32 The literature reports that another potential mechanism for pain in the post-COVID period is the long period of stay in the ICU; however, in our study, none of the participants required intensive care, which may reinforce the mechanism of the direct impact of the virus on the peripheral nervous system or central nervous system or induce post-viral immune syndrome. 33
Weight maintenance impairment was more common during the first 3-6 months, mainly in patients with anosmia and dysgeusia. After the COVID-19 pandemic, many patients have experienced weight loss, muscle loss, and malnutrition. Moreover, almost all patients with COVID-19 have 1 or more nutritional complaints, such as decreased appetite, feeling full, shortness of breath, altered taste, and loss of taste. 34 Several of these symptoms are associated with reduced nutrient intake, increased energy expenditure, and decreased nutrient absorption. 35 Wierdsma et al. suggested that nutritional problems persist after COVID-19, indicating that all patients require prolonged nutritional support and monitoring. 35
Muscle power and muscle tone were also impaired at 6-12 months, particularly in patients with walking difficulties. Musculoskeletal disorders and reduced muscle strength have been observed in critically ill patients with prolonged hospitalization; 36 however, none of our patients required intensive care hospitalization. Fatigue and myalgia are the main musculoskeletal symptoms described in the literature in mildly and non-critically ill patients with COVID-19. There are 2 hypotheses suggesting different mechanisms of action of the virus on the skeletal muscle tissue: 37 (1) SARS-CoV-2 binds to the ACE2 receptor on the skeletal muscle cell surface, and (2) elevates inflammatory processes in the musculoskeletal tissue. Recent studies have suggested that musculoskeletal symptoms can persist for weeks and/or months, giving rise to a debilitating condition known as post-COVID condition.38-42
Respiratory and cardiovascular system impairments were particularly observed in patients with walking difficulties. These impairments seem to be recurrent in post-COVID-19 individuals.13,43 A reduced ability to perform activities of daily living and moderate dyspnea at rest were observed even in patients with mild disease who did not progress to acute respiratory failure. 44 In addition, individuals with post-mild COVID-19 have reported long-term persistence of fatigue and dyspnea. 45 Fatigue is frequent in patients with COVID-19, regardless of the degree of severity, and is accentuated in cases of associated chronic diseases and an acute inflammatory state of systemic infection by SARS-CoV-2. 2 Walking difficulties can reduce physical activity levels and lead to cardiorespiratory complications in the post-COVID condition.
Activity limitations and participation restrictions
Watching and listening restrictions were associated with brain fog at 3-6 months and dizziness and headache at 6-12 months. Restrictions related to watching may be associated with visual changes caused by the vascular and inflammatory processes.28-31 However, viral infections can also affect the auditory system, thus limiting listening tasks. What appears to be slightly more common is hearing loss, tinnitus, or dizziness later in the process. 46 Both visual and hearing losses can negatively interfere with watching and listening tasks. However, periods of social isolation, mobility restrictions, and cognitive changes can also limit these activities.
Restrictions in undertaking multiple tasks were associated with fatigue, irritability, brain fog, and walking difficulty. Luvizutto et al. showed that choice reaction time can be decreased after COVID-19 diagnosis over 1 year, and this alteration was associated with decreased cognitive function during multiple tasks. 47 Some studies have shown that COVID-19 can also alter the brain’s functional connectivity pattern, causing brain fog and cognitive dysfunction for months after infection resolution.11,21,48 Loss of executive function, reduced sustained attention, and slow reasoning for motor responses during everyday tasks in COVID-19 patients can lead to difficulties in performing multiple tasks. Additionally, most COVID-19 patients experience persistent muscle symptoms, such as fatigue and walking difficulty, that decrease their ability to perform activities of daily living, 37 such as fatigue and walking difficulty.
Difficulties in lifting and carrying objects, fine hand use, and walking have been reported mainly in individuals with fatigue, irritability, and difficulty walking. Such limitations are common in individuals with decreased muscle tone and strength,13,49 impairing the practice of physical exercise, and leading to muscle deconditioning and fatigue.50,51 Indeed, COVID-19 patients suffer from multiple symptoms, and acute care takes place in strict isolation, which reduces a patient's mobility, 52 and can impact long-term functioning.
Driving restrictions are associated with brain fog and fatigue. Driving requires high demands linked to attention and concentration. 53 Another factor is that both driving fatigue and distraction are manifestations of insufficient attention being allocated to driving tasks. 54 Yan et al. demonstrated the correlation between driving performance and executive control function. 54
Limitations and future directions
The limitations of this study include the lack of information regarding the history of symptoms prior to acute COVID-19 infection and details on the severity of symptoms. It is also noteworthy that ICF was not used for the entire spectrum. The ICF checklist was developed as a generic tool and not specific to any health condition. Thus, it may not have captured all issues associated with post-COVID condition. In addition, this was a single-center study with a relatively small sample size for the study design, and without a control group of asymptomatic patients; thus, the generalizability of the results is narrow. Fortunately, although the pandemic has passed, some viral variants still affect health and clinical care. This study provides a natural history and possible clinical and functional evolution of COVID-19 over 1 year and may help prevent and/or alleviate long-term complications.
Conclusion
Individuals with mild-to-moderate COVID-19 showed impairments in structure and body function, activity limitations, and participation restrictions during the 1-year follow-up period. Impairments were higher during the 6- to 12-month period and may be monitored using the ICF classification. Our results will stimulate the development of future core set for post-COVID condition.
Supplemental Material
Supplemental Material for Post-coronavirus disease 2019 functional impairments, limitations, and restrictions: A prospective cohort study based on the international classification of functioning, disability, and health by Isabella Polo Monteiro, Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Rodrigo Bazan, Luciane Aparecida Pascucci Sande de Souza, and Gustavo José Luvizutto in Journal of Central Nervous System Disease
Acknowledgements
This work was supported by the Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel, and Ministry of Science, Technology, Innovations, and Communications (MCTIC). (Process 401192/2020-2).
Author contributions: 1. Made substantial contributions to the concept or design of the work: IPM, RB, LAPSS, and GJL 2. Acquisition, analysis, or interpretation of data: PAP, ATS, KSMBS 3. Drafted the article or revised it critically for important intellectual content: IPM, LAPSS, and GJL 4. Approved the version to be published: IPM, PAP, ATS, KSMBS, RB, LAPSS and GJL
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Grants were received from the National Council for Scientific and Technological Development (CNPq); Ministry of Science, Technology, Innovations, and Communications (MCTIC); and CNPq (Process 401192/2020-2).
Ethical approval: This study was approved by the Research Ethics Committee of the Federal University of Triângulo Mineiro (UFTM) (CAAE: 30684820.4.0000.5154). Participants signed an informed consent form to participate in the study.
Supplemental Material: Supplemental material for this article is available online
ORCID iD
Gustavo Jose Luvizutto https://orcid.org/0000-0002-6914-7225
Data Availability Statement
All Data used to write up this manuscript is available for review upon a reasonable request.
References
- 1.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2021;384(5):481-483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosino P, Papa A, Maniscalco M, et al. COVID-19 and functional disability: current insights and rehabilitation strategies. Postgrad Med. 2021;97(1149):469-470. doi: 10.1136/postgradmedj-2020-138227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015-2016. doi: 10.1001/jama.2021.5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitacca M, Migliori GB, Spanevello A, et al. Management and outcomes of post-acute COVID-19 patients in Northern Italy. Eur J Intern Med. 2020;78:159-160. doi: 10.1016/j.ejim.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings G, Monaghan A, Xue F, et al. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10:5913. doi: 10.3390/jcm10245913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization [Internet] . Country & Technical Guidance - Coronavirus disease (COVID-19). Geneva: WHO; 2023. [cited 2023 Apr 20]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance [Google Scholar]
- 7.ICF International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 8.Maribo T, Petersen KS, Handberg C, et al. Systematic literature review on ICF from 2001 to 2013 in the Nordic countries focusing on clinical and rehabilitation context. J Clin Med Res. 2016;8(1):1-9. doi: 10.14740/jocmr2400w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nopp S, Moik F, Klok FA, et al. Outpatient pulmonary rehabilitation in patients with long covid improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101(6):593-601. doi: 10.1159/000522118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palau P, Domínguez E, Gonzalez C, et al. Effect of a home-based inspiratory muscle training programme on functional capacity in postdischarged patients with long COVID: the InsCOVID trial. BMJ Open Respir Res. 2022;9(1):e001439. doi: 10.1136/bmjresp-2022-001439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman B, Bluemke DA, Lüscher TF, et al. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157-1172. doi: 10.1093/eurheartj/ehac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox SE, Heide RSV. COVID-19: the heart of the matter-pathological changes and a proposed mechanism. J Cardiovasc Pharmacol Therapeut. 2021;26(3):217-224. doi: 10.1177/1074248421995356 [DOI] [PubMed] [Google Scholar]
- 13.Hugon J, Msika EF, Queneau M, et al. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44-46. doi: 10.1007/s00415-021-10655-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-1032. doi: 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 15.Andrenelli E, Negrini F, de Sire A, et al. Rehabilitation and COVID-19: a rapid living systematic review 2020 by Cochrane rehabilitation field. Update as of september 30th, 2020. Eur J Phys Rehabil Med. 2020;56(6):846-852. doi: 10.23736/S1973-9087.20.06672-1 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence (NICE) . COVID-19 rapid guideline: managing the long-term effects of COVID-19. London, UK: National Institute for Health and Care Excellence (NICE); 2020. [PubMed] [Google Scholar]
- 17.Berzina G, Sveen U, Paanalahti M, et al. Analyzing the modified rankin scale using concepts of the international classification of functioning, disability and health. Eur J Phys Rehabil Med. 2016;52(2):203-213. [PubMed] [Google Scholar]
- 18.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25(4):289-304. doi: 10.1097/WAD.0b013e318211c83c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostanjsek N. Use of the international classification of functioning, disability and health (ICF) as a conceptual framework and common language for disability statistics and health information systems. BMC Publ Health 2011; 11(Suppl 4): S3. DOI: 10.1186/1471-2458-11-S4-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewert T, Fuessl M, Cieza A, et al. Identification of the most common patient problems in patients with chronic conditions using the ICF checklist. J Rehabil Med 2004;(44 Suppl):22-29. doi: 10.1080/16501960410015362. [DOI] [PubMed] [Google Scholar]
- 21.Selb M, Stucki G, Li J, et al. On behalf of the ISPRM ClinFIT Task Force‡. Developing clinfit COVID-19: an initiative to scale up rehabilitation for COVID-19 patients and survivors across the care continuum. J Int Soc Phys Rehabil Med. 2021;4:174-183. [Google Scholar]
- 22.Norrefalk JR, Borg K, Bileviciute-Ljungar I. Self-scored impairments in functioning and disability in post-COVID syndrome following mild COVID-19 infection. J Rehabil Med 2021; 53: jrm00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7(7):611-627. doi: 10.1016/S2215-0366(20)30203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697-707. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guedj E, Campion JY, Dudouet P, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imag. 2021;48(9):2823-2833. doi: 10.1007/s00259-021-05215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022:434:120162. doi: 10.1016/j.jns.2022.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.COVID-19 Mental Disorders Collaborators . Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700-1712. doi: 10.1016/S0140-6736(21)02143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tohamy D, Sharaf M, Abdelazeem K, et al. Ocular manifestations of post-acute COVID-19 syndrome, upper Egypt early report. J Multidiscip Healthc. 2021;14:1935-1944. doi: 10.2147/JMDH.S323582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savastano MC, Larici AR, Crincoli E, et al. Retinal vascular impairment matched to the pulmonary damage in early post-COVID 19 patients. Eur J Ophthalmol. 2022;32:11206721221079153. doi: 10.1177/11206721221079153 [DOI] [PubMed] [Google Scholar]
- 30.Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol. 2020;55(4):e125-e129. doi: 10.1016/j.jcjo.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siedlecki J, Brantl V, Schworm, et al. COVID-19: ophthalmological aspects of the SARS-CoV 2 global pandemic. Klin Monbl Augenheilkd. 2020;237(5):675-680. doi: 10.1055/a-1164-9381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bittencourt JV, Reis FJJ, Nogueira LAC. Pain in COVID-19 patients: a call to action for physical therapists to provide pain management after an episode of COVID-19. Braz J Phys Ther. 2021;25(4):367-368. doi: 10.1016/j.bjpt.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Attal N, Martinez V, Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6(1):e884. doi: 10.1097/PR9.0000000000000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fedele D, De Francesco A, Riso S. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition. 2021;81:111016. doi: 10.1016/j.nut.2020.111016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierdsma NJ, Kruizenga HM, Konings LA, et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021;43:369-376. doi: 10.1016/j.clnesp.2021.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paneroni M, Simonelli C, Saleri M, et al. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil. 2021;100(2):105-109. doi: 10.1097/PHM.0000000000001641 [DOI] [PubMed] [Google Scholar]
- 37.Dos Santos PK, Sigoli E, Bragança LJG, et al. The musculoskeletal involvement after mild to moderate COVID-19 infection. Front Physiol. 2022;13:813924. doi: 10.3389/fphys.2022.813924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brüssow H, Timmis K. COVID-19: long covid and its societal consequences. Environ Microbiol. 2021;23(8):4077-4091. doi: 10.1111/1462-2920.15634 [DOI] [PubMed] [Google Scholar]
- 39.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 40.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Publ Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall M. The four most urgent questions about long COVID. Nature. 2021;594(7862):168-170. doi: 10.1038/d41586-021-01511-z [DOI] [PubMed] [Google Scholar]
- 42.Salamanna F, Veronesi F, Martini L, et al. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. a systematic review of the current data. Front Med. 2021;8:653516. doi: 10.3389/fmed.2021.653516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González J, Benítez ID, Carmona P, et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187-198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zampogna E, Migliori GB, Centis R, et al. Functional impairment during post-acute COVID-19 phase: preliminary finding in 56 patients. Pulmonology. 2021;27(5):452-455. doi: 10.1016/j.pulmoe.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carfì A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol. 2021;60(12):935-945. doi: 10.1080/14992027.2021.1896793 [DOI] [PubMed] [Google Scholar]
- 47.Luvizutto GJ, Sisconetto AT, Appelt PA, et al. Can the choice reaction time be modified after COVID-19 diagnosis? A prospective cohort study. Deme Neuropsyc. 2022;16(3):354-360. do: 10.1590/1980-5764-dn-2021-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg E, Podell K, Sodickson DK, et al. The brain after COVID-19: compensatory neurogenesis or persistent neuroinflammation? EClinicalMedicine. 2020;31:100684. doi: 10.1016/j.eclinm.2020.100684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baratto C, Caravita S, Faini A, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985). 2021;130(5):1470–1478. doi: 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinaldo RF, Mondoni M, Parazzini EM, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58(2):2100870. doi: 10.1183/13993003.00870-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020;56(4):2002096. doi: 10.1183/13993003.02096-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steardo L, Steardo L, Zorec R, et al. Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020;229(3):e13473. doi: 10.1111/apha.13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patoine A, Mikula L, Mejía-Romero S, et al. Increased visual and cognitive demands emphasize the importance of meeting visual needs at all distances while driving. PLoS One. 2021;16(3):e0247254. doi: 10.1371/journal.pone.0247254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan L, Wen T, Zhang J, et al. An evaluation of executive control function and its relationship with driving performance. Sensors. 2021;21(5):1763. doi: 10.3390/s21051763 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Post-coronavirus disease 2019 functional impairments, limitations, and restrictions: A prospective cohort study based on the international classification of functioning, disability, and health by Isabella Polo Monteiro, Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Rodrigo Bazan, Luciane Aparecida Pascucci Sande de Souza, and Gustavo José Luvizutto in Journal of Central Nervous System Disease
Data Availability Statement
All Data used to write up this manuscript is available for review upon a reasonable request.