Abstract
Infraspinatus tendon is the most affected tendon of the rotator cuff, being an important posterior component of the shoulder joint. Hyperlipidemia is a predisposing factor in the progression of rotator cuff tears and retear. We studied the effect of hyperlipidemia on the biomechanical properties of rotator cuff tendons. The infraspinatus tendon of the rotator cuff from hyperlipidemic swine were collected and tested for ultimate tensile strength (UTS) and modulus of elasticity. Dynamic mechanical analysis was performed to examine viscoelastic properties. The findings revealed no significant difference in UTS but had significantly lower modulus of elasticity in the infraspinatus tendon of the hyperlipidemic group compared to the control group. Moreover, differences in the dynamic modulus, storage modulus, and loss modulus were not statistically significant between the hyperlipidemic and control swine. There was no difference in water content between the groups but the hyperlipidemic group had fatty infiltration aiding the initial decrease in mechanical properties. These findings suggest an association between fat deposition and early changes in the biomechanical properties of the tendons in the shoulder rotator cuff in hyperlipidemic state.
Keywords: Biomechanics, Dynamic modulus, Hyperlipidemia, Infraspinatus tendon, Mechanical properties, Rotator cuff
1. Introduction
The force generated by the muscles of the rotator cuff are transferred to the bone by its tendons. Injuries and tears on the rotator cuff tendons compromise the normal function and in turn may lead to surgeries. Infraspinatus tendon is the most frequently affected tendon being the stabilizer of forces in the posterior side [1]. These impact the quality of life in addition to the economic burden. The prevalence of rotator cuff injuries is 2.8–15 % leading to surgical repair with a failure rate of 20–94% [2–4]. These tendons are formed by the spatial arrangement of the fascicles bundle bound by the interfascicular matrix where the fascicles are formed by collagen fibers [5,6]. Researchers are investigating to understand the underlying cause and progression of these tears. Age, physical activities, duration of symptoms, diabetes, smoking are factors that affect the effective healing of rotator cuff injuries. Recently, there is a lot of interest in studying the association of hyperlipidemia in rotator cuff injuries and its onset on treatments methodologies.
Hyperlipidemia, a systemic metabolic disease, is well known for its impact on vascular system, but it equally affects the musculoskeletal system impacting the strength and range of motion [7,8]. More than 100 million adults in the United States are reported to show hyperlipidemia and hence the associated co-morbidities, including cardiovascular events and heart failure, are on the rise. Hyperlipidemia is reported to accumulate lipids in the ligaments and tendons [3]. In clinical studies, the association of hyperlipidemia with rotator cuff injuries and tears is inconsistent [3,9]. The mechanical properties of rotator cuff tendons of hyperlipidemic rats were reported earlier. Mechanical properties of the tendons and ligaments are compromised under hyperlipidemic conditions [10].
Previously we have reported that hyperlipidemia in swine models have induced pathological changes in the organization of the tendon, extracellular matrix (ECM), and cellularity [4]. We aimed to study the changes in mechanical properties of the rotator cuff tendon swine under hyperlipidemic diet. In this study we investigated the changes in the mechanical properties and viscoelastic properties of the infraspinatus tendon on hyperlipidemic swine.
2. Materials and Methods
2.1. Animals and tendon tissue collection and preparation
The Institutional Animal Care and Use Committee (IACUC) of Western University of Health Sciences, Pomona, CA, USA approved the experimental research protocol (R22IACUC034).
Female Yucatan miniswine (Sus scrofa) weighing 25–30 kg, were purchased from Premier Bioresources, Ramona, CA, USA. Swine were acclimatized to 12/12 hours of light-dark cycle and were fed twice every day with either regular pig diet (Group-I) or high-cholesterol-high-fat diet to develop hyperlipidemia (Group II) [4,11]. After 40 weeks the animals were sacrificed, and the infraspinatus tendon tissues of the rotator cuff were collected and stored at −80°C until testing. On the day of testing the tendon tissues were thawed using a two-step protocol: (i) 4 h at 4°C, and (ii) 2 h at room temperature [12]. After thawing, two equal pieces from the tendon tissue were manually cut from the tendon core using scalpel blade. The cut pieces were approximately 55 mm in length for tensile testing and Dynamic mechanical analysis (DMA) and kept at 4°C to maintain the freshness of tissue until testing.
2.2. Tensile strength and strain
Tensile test is a destructive testing that measures the force required to break the sample material and the elongation of the sample material at that breaking point. The tensile testing was conducted on 6 tendons from control animals and 8 tendons from hyperlipidemic animals. Approximately 55 mm of the tendon core were cut, and the length, width and thickness were measured using digital vernier caliper. The smaller dimensions were used in calculating the cross-sectional area. The tendons were secured in between sandpaper and mounted using the tension grips on to TA Electroforce 3300 (TA Instruments, New Castle, DE, USA) equipped 1000 N load cell. The gauge length was 30 mm. The samples were preconditioned using for 10 cycles at a frequency of 1.0 Hz between 0 and 10 N under load control [13]. Following to preconditioning, the tendon tissue samples were ramped to failure at a crosshead speed set to 100μm/s while recording the force and displacement continuously. Failure was defined as the decrease in load below 20% of the maximum load. The engineering stress (σ) and engineering strain (ε) were determined, and the stress-strain curve was plotted. The modulus of elasticity (E) was obtained from the slope of the linear region of the stress-strain curve. The ultimate tensile strength (UTS) and the strain at failure were also calculated [14,15].
2.3. Dynamic mechanical analysis
Dynamic mechanical analysis (DMA) is a basic tool used to measure the viscoelastic properties of materials. A small oscillating force is applied (stress) and the strain is measured, and the viscoelastic properties are determined in relation to temperature, time, or frequency. For dynamic mechanical analysis (DMA) 6 tendons from Group I–animals with regular diet and 8 tendons from Group II–hyperlipidemic diet. Approximately 55 mm of the tendon core were cut, and the length, width and thickness were measured using digital vernier caliper. The tendons were secured in between sandpaper and mounted using the tension grips on to TA Electroforce 3300 (TA Instruments, New castle, DE, USA) equipped 1000 N load cell. An initial load of 2.0 N was applied to remove any slack and the gauge length was recorded. The tendon tissues were preconditioned with 10 cycles at a frequency of 1.0 Hz between 0 and 10 N under load control. Sinusoidal loading between 10 N and 20 N was applied and two frequency sweep was performed at (i) 0.2 Hz to 2.0 Hz with an increase in frequency of 0.2Hz until 2 Hz, and (ii) 1 Hz to 41 Hz with increase in frequency of 10 Hz until 41 Hz. Throughout the testing, the samples were kept immersed in PBS at room temperature to prevent excess drying. The viscoelastic properties, dynamic modulus (E*), storage modulus (E’), loss modulus (E”) and damping ability (Tan δ) were calculated and recorded by the machine [12,13].
2.4. Water content
The water content of the tendon core (10 mm length, 3–4 mm width and thickness) was calculated based on the weight of tendon tissues before (Wwet) and after drying (Wdry) for 72 hours at 40°C. The samples were weighed using analytical balance with the resolution of 0.01 mg. The water content was calculated using the below formula:
2.5. Histology
Tendon tissue samples were fixed with 10% buffered formalin for 72 hours, processed, embedded in paraffin, and 7 μm thick sections were cut. The sections were stained with hematoxylin and eosin (H&E) and analyzed for cellularity, ECM organization, and tendon alignment.
2.6. Statistical analysis
The statistical analyses for tensile testing and modulus of elasticity were done using unpaired Student t-test and dynamic mechanical analysis were calculated using two-way analysis of variance (ANOVA) using GraphPad Prism.9.5.1 software. The p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Tensile strength
The ultimate tensile strength (UTS), failure strain percentage, and the modulus of elasticity (E) are shown in Figure 1. There was no significant difference in the mean value of the UTS between the control group receiving normal pig diet and the hyperlipidemic group (Figure 1A). However, the modulus of elasticity was significantly decreased in the hyperlipidemic animals compared to the control group (Figure 1B). The failure strain percentages did not have statistically significant difference between the control swine and the hyperlipidemic swine (Figure 1C).
Figure 1:
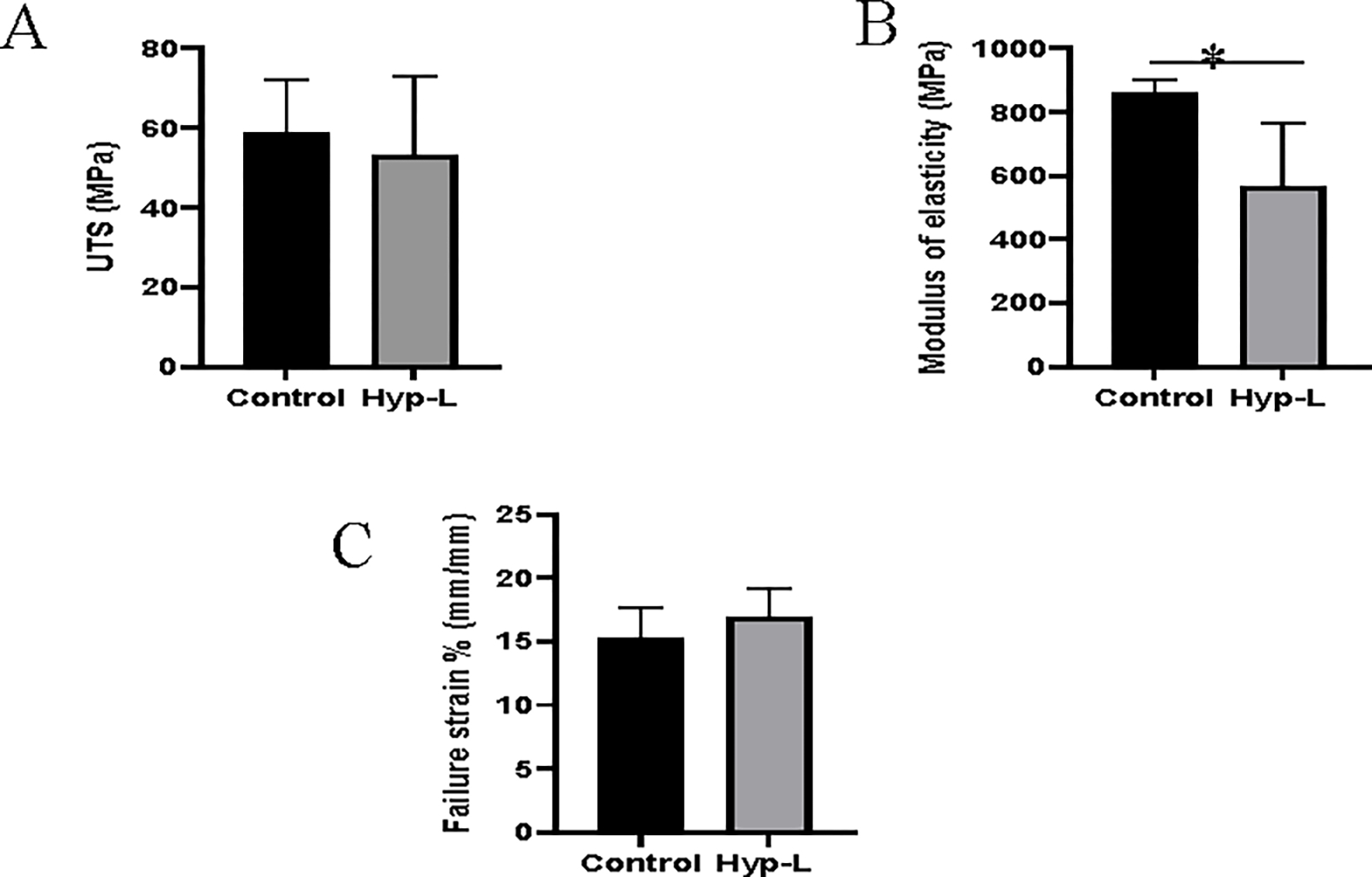
Mechanical properties of tendon tissues: (A) Ultimate tensile strength, (B) Modulus of elasticity, (C) Failure strain %. Control indicates the group of animals that received normal diet (group 1), Hyp-L indicates the group of animals that received high-cholesterol-high-fat diet (Group 2). Values are shown as mean ± SD; n=6–8). * Indicates significant difference (p<0.05, student-t test).
3.2. DMA and water content
The viscoelastic properties recorded from the dynamic mechanical analysis at low frequencies are shown in Figure 2. The dynamic modulus and storage modulus increased with increase in frequency while the loss modulus decreased with increase in frequency. No significant difference in dynamic modulus, loss modulus, or damping ability was observed between the two groups in the respective low frequencies tested (0.2–2 Hz) (Figure 2) and at higher frequencies tested (Figure 3). The control group showed a more viscous nature than the hyperlipidemic group at low frequencies. The water content of tendons from the control group (65.52 ± 4.55 %) and the hyperlipidemic group (65.60 ± 4.10 %) were not different (Table 1).
Figure 2:
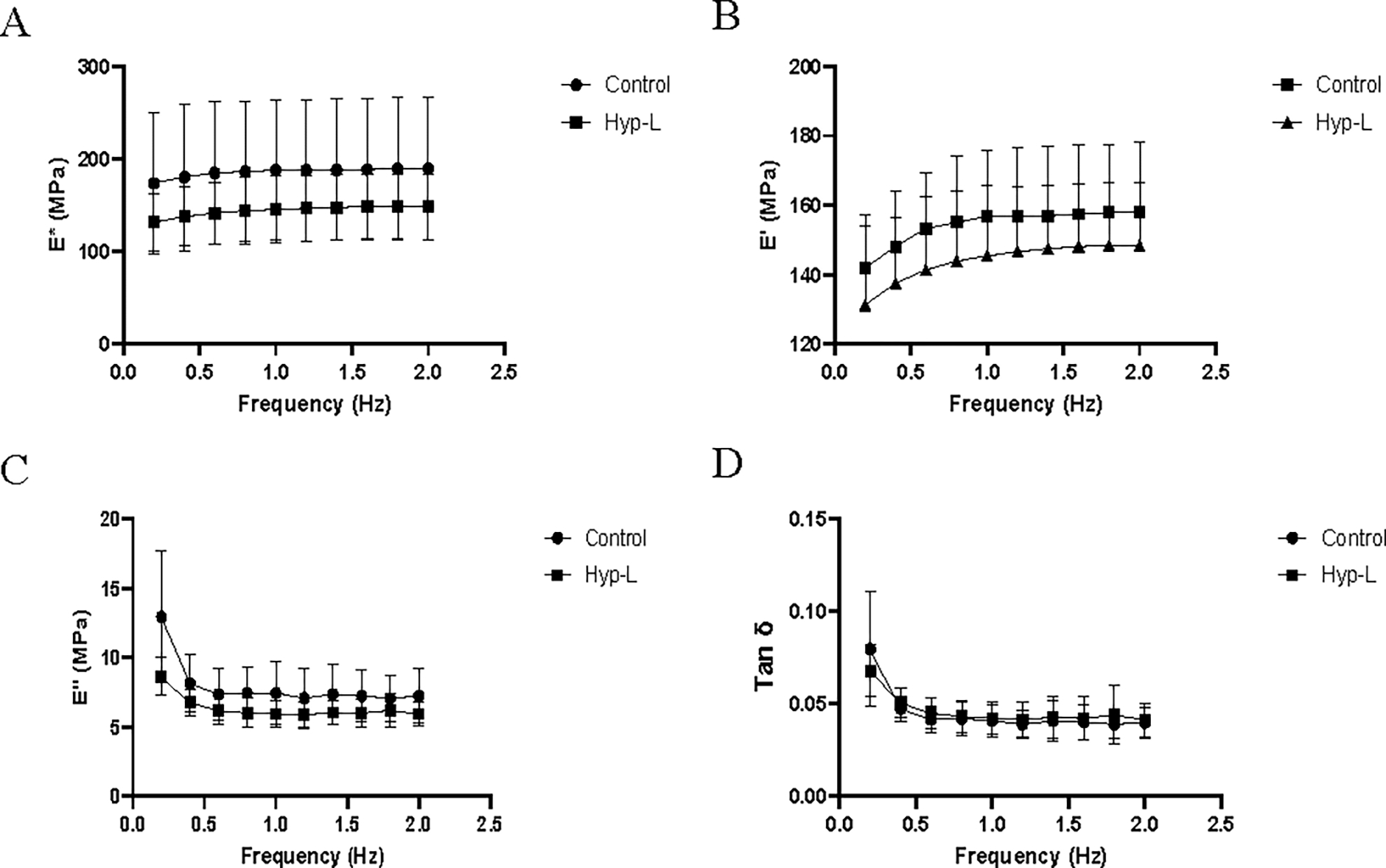
Viscoelastic properties of the tendon tissues at low frequencies: (A) dynamic modulus (E*), (B) storage modulus (E’), (C) loss modulus (E”) and (D) damping ability (Tan δ). Control indicates the group of animals that received normal diet, Hyp-L indicates the group of animals that received high-cholesterol-high-fat diet. Values are shown as mean ± SD and 95 % CI; n=6–8).
Figure 3:
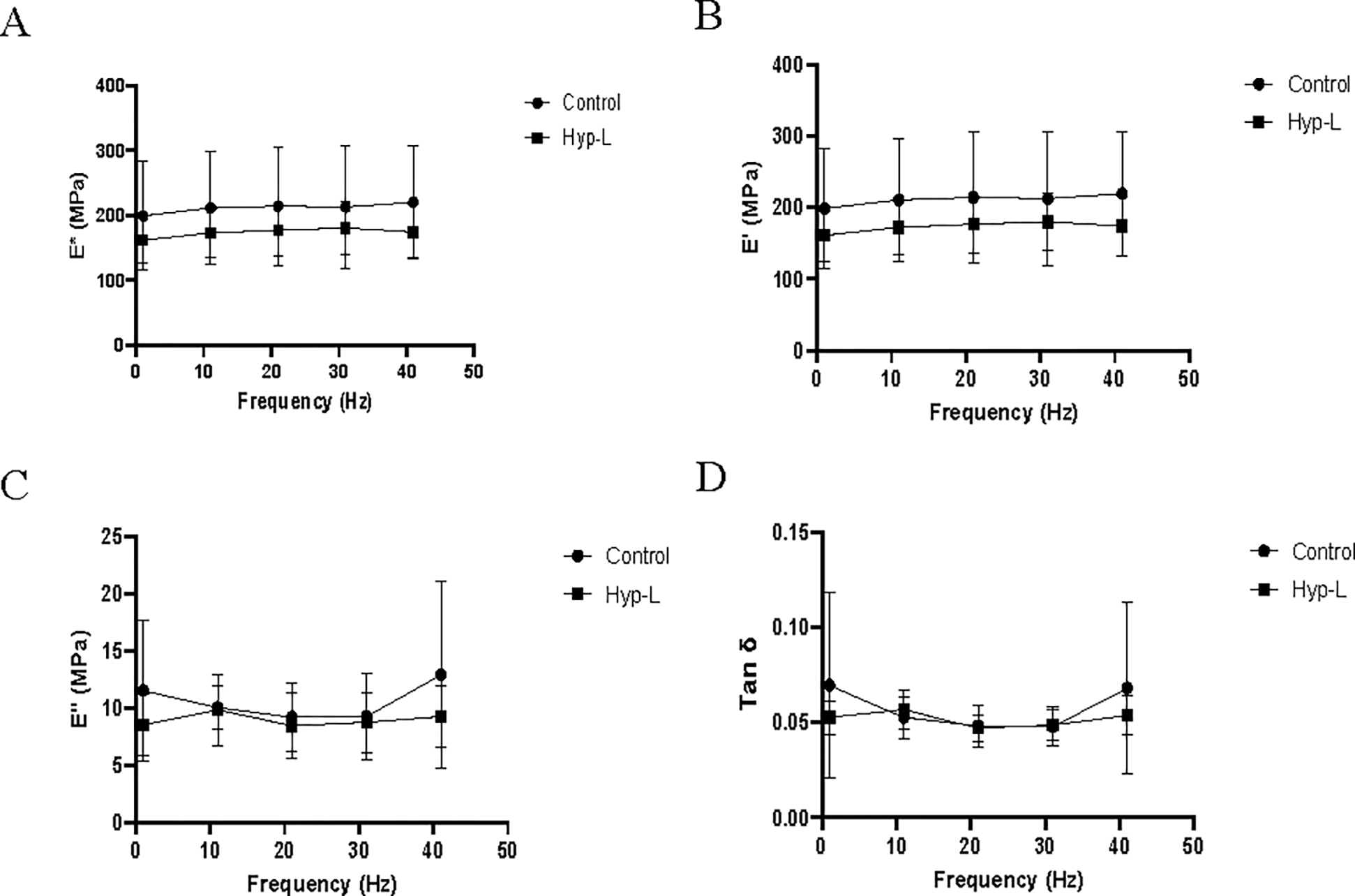
Viscoelastic properties of the tendon tissues at higher frequencies: (A) dynamic modulus (E*), (B) storage modulus (E’), (C) loss modulus (E”) and (D) damping ability (Tan δ). Control indicates the group of animals that received normal diet, Hyp-L indicates the group of animals that received high-cholesterol-high-fat diet. Values are shown as mean ± SD and 95 % CI; n=6–8).
Table 1:
Water content of tendons of the control group and hyperlipidemic group.
| Control | Hyperlipidemic group | |
|---|---|---|
| % Water content (wt/wt) | 65.52 ± 4.55 | 65.60 + 4.10% |
3.3. Histology
The representative image of H&E staining of the tendon tissue is shown in Figure 4. The control group had longitudinally arranged fibers with the presence of decreased cells. The cells are presented with elongated nuclei, a characteristic of mature tenocytes. On the other hand, the hyperlipidemic group showed disorganized extracellular matrix (ECM) and increased infiltration of inflammatory cells and proliferating teloblast like cells. The hyperlipidemic group also showed fat deposition in the tendons.
Figure 4:
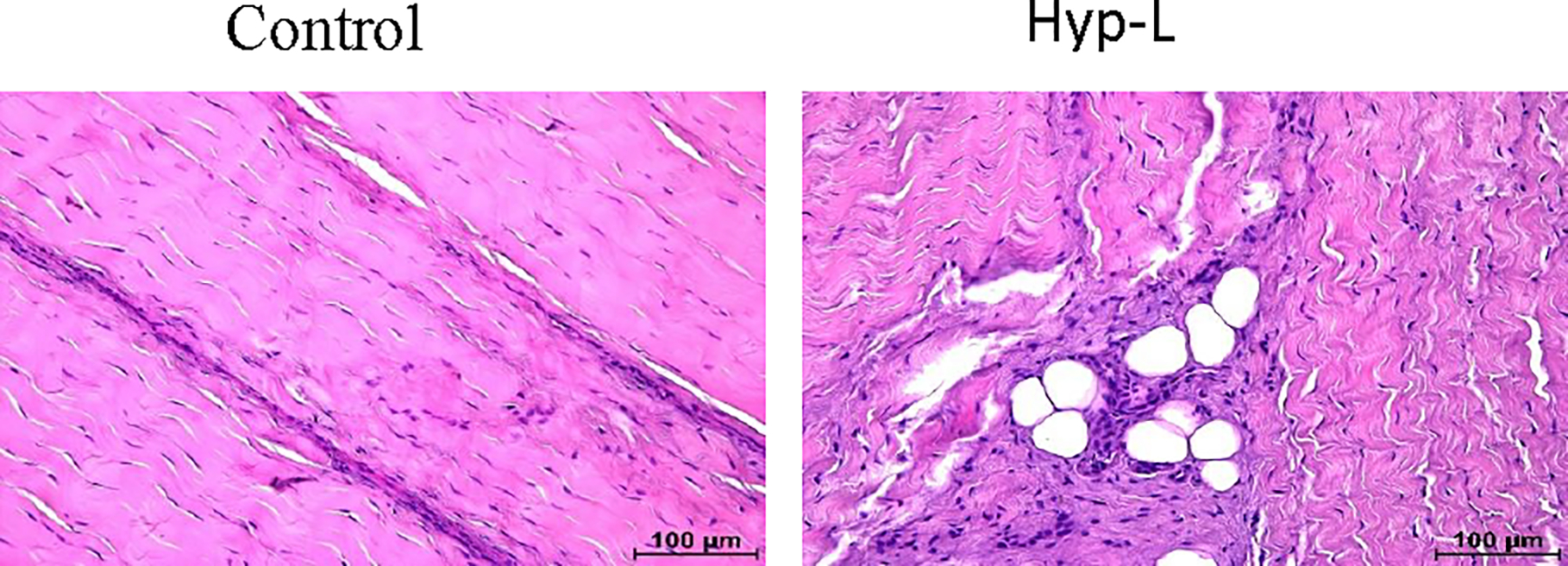
Representative image of tendon histology (H&E). Control indicates the group of animals that received normal diet (group 1), Hyp-L indicates the group of animals that received high-cholesterol-high-fat diet (Group 2). The images were acquired in 40x magnification.
4. Discussion
Hyperlipidemia is a risk factor for number of comorbid conditions such as hypertension, diabetes, heart failure [16]. Hyperlipidemia is also associated with the accumulation of lipids within the extracellular matrix of the tendon and hence can reduce the mechanical properties of the tendon [3]. Hence, mechanical testing of the tendon tissues is important in understanding the inherent strength and viscoelastic properties that are responsible for the biological functions. We studied the differences in mechanical properties of the infraspinatus tendon of the rotator cuff in swine that received normal diet and swine that received hyperlipidemic diet. The ultimate tensile strength of the control and the hyperlipidemic group were not statistically significant. Similarly, failure strain % also did not have a significant difference between the two groups as expected. The modulus of elasticity (E) calculated from the linear portion of the stress-strain curve was lower in the hyperlipidemic group of animals when compared to the control group and is statistically significant (p=0.03) when analyzed with a student-t test. Our results varied from the previous studies conducted on supraspinatus tendon of hyperlipidemic mouse rat and monkey where they have observed an increase in the tensile strength and the modulus of elasticity [17]. Moreover, we have collected the tendon tissues at a mean age of 40 weeks, and this might be a contributing factor for having similar tensile strength. Age is a factor that would have contributed factor to this trend. Meanwhile the tensile modulus or the modulus of elasticity has decreased in the hyperlipidemic group as expected, stating there are other factors contributing to the decrease of modulus of elasticity than age factor. The collagen content in the tendons decreases with increase in age. In an earlier study where the collagen content and tensile modulus were analyzed among older me (age 67 ± 3 yr) and younger men (age 27 ± 2 yr) with similar physical activities it was observed there was a decrease in the collagen content but no significant difference in the tensile modulus. But the loss of collagen was balanced by the inter and intra molecular crosslinking of collagen in older men which was less pronounce in younger men [18]. The linear range of the tendon extension is caused by the sliding of the collagen fibers. Hence, age could not be the decisive factor in causing the difference in tensile modulus between the normal and hyperlipidemic group of animals. Mechanical properties of tendons decrease with age [19,20], physical activities and comorbid conditions [21]. Hyperlipidemia could be a contributing factor in the lowered of mechanical properties in the hyperlipidemia group of animals.
The dynamic mechanical analysis was performed in the linear range of the tendon extension just above the toe region. The low frequency ranges (0.2 −2.0 Hz) in our study showed no significant differences in viscoelastic properties of the tendons of the hyperlipidemic group compared to the control group animals. The viscoelastic properties give the strain dependent dynamic mechanical behavior of biological tissues. The viscoelastic properties of soft tissues increase with decrease in water content of the tissues [13]. Dynamic mechanical analysis was carried out in a PBS bath to reduce and make sure there is now drying of tissues which affects the viscoelastic properties while testing. Decrease in water content in of the tissues will enhance the mechanical properties of soft tissues such as tendons and ligaments [22,23]. We observed the water content of the tendon tissue samples from the control group (65.60 ± 4.18) and the hyperlipidemic group of animals (65.52 ± 4.55) was not significantly different. The extension of tendons in response to load is characterized by (1) an initial uncoiling of collagen fibers, (2) sliding of fibers and fibrils along the linear region, and (3) stretching of the tropocollagen backbone until final rupture of the fibril. The inter and intra collagen enzymatic crosslinking formed initially are immature divalent bonds which over time form trivalent bonds [18,24–26].
The H&E staining of the sections of the hyperlipidemic group of animals showed infiltration of fat in the tendon tissues and inflammatory cells in the tendon tissue, which was not present in the control tendon tissues. Injury and remodeling of the tendons have recruitment of actively dividing teloblasts and inflammatory cells to the site of injury [27,28]. We have earlier shown that hyperlipidemia increases fat depositions in swine [29–31] and is a predisposing factor for the risk of rotator cuff tear and reinjury [32,33]. The fatty infiltration in the tendon tissues would have contributed to the lowered tensile modulus with no changes in the viscoelastic properties. Infiltration of fat in the tendon would have contributed to the reduced modulus of elasticity, but it would not have altered the inter and intra bonds between the collagen fibers which is expected as the deposition of fat is increased. And hence the collagen sliding during the extension in the initial region of the linear phase of extension would have been unaffected and as the deposition increases would bring differences in viscoelastic properties. In our study we observed the lowering of tensile modulus in the hyperlipidemic swine. There may be other factors also involved in weakening of collagen fibrils of the tendons that need to be studies. Long term studies would give insights on the changes in tensile strength as the increase in fatty infiltration is higher with increase in age and to study the force transferred by the tendons.
5. Conclusion
This study reveals the early onset of changes in the dynamic mechanical properties with decreased modulus of elasticity and viscoelastic properties of the infraspinatus tendons in hyperlipidemic swine. However, long-term studies would be required to elucidate the changes in the tensile strength as the fatty infiltration would be more pronounced with age and other predisposing factors.
6. Limitations
The tendon tissues at the time of tissue collection were relatively young and the sample size was small. Also, the mechanical properties vary with variation in physical activities. The measurement of extension was calculated from the crosshead displacement of clamps to compute the strain values. Measurement with video extensometer would have provided better information on the parameters. Moreover, the variation in the physical activities warrants further investigation in detail as the swine were allowed to walk freely in the pen.
Funding:
The research work of DKA is supported by the R01 HL144125 and R01 HL147662 grants from the National Institutes of Health, USA. The content of this critical review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interest: All the authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Competing interests: The authors declare no competing interests.
Consent for publication: All the authors have read the manuscript and consented for publication.
References
- 1.Kronberg M, Németh G, Broström LA. Muscle activity and coordination in the normal shoulder. An electromyographic study. Clin Orthop Relat Res (1990): 76–85. [PubMed] [Google Scholar]
- 2.Agrawal DK, Dougherty K, Dilisio M. Vitamin D and the immunomodulation of rotator cuff injury. J Inflamm Res 9 (2016): 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Qu J. The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg Res 13 (2018): 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang W, Sekhon S, Teramoto D, et al. Pathological alterations in the expression status of rotator cuff tendon matrix components in hyperlipidemia. Mol Cell Biochem 478 (2023): 1887–1898. [DOI] [PubMed] [Google Scholar]
- 5.Thorpe CT, Birch HL, Clegg PD, et al. The role of the non-collagenous matrix in tendon function. Int J Exp Pathol 94 (2013): 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thankam FG, Dilisio MF, Gross RM, et al. Collagen I: a kingpin for rotator cuff tendon pathology. Am J Transl Res 10 (2018): 3291–3309. [PMC free article] [PubMed] [Google Scholar]
- 7.Mundi S, Massaro M, Scoditti E, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 114 (2018): 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazdani AN, Rai V, K Agrawal DK. Rotator Cuff Health, Pathology, and Repair in the Perspective of Hyperlipidemia. Journal of Orthopaedics and Sports Medicine 04 (2022): 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin TTL, Lin CH, Chang CL, et al. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 43 (2015): 2126–2132. [DOI] [PubMed] [Google Scholar]
- 10.Matson AP, Kim C, Bajpai S, et al. The effect of obesity on fatty infiltration of the rotator cuff musculature in patients without rotator cuff tears. Shoulder Elbow 11 (2019): 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thankam FG, Wilson VED, Radwan MM, et al. Involvement of ischemia-driven 5-lipoxygenase-resolvin-E1-chemokine like receptor-1 axis in the resolution of post-coronary artery bypass graft inflammation in coronary arteries. Mol Biol Rep 49 (2022): 3123–3134. [DOI] [PubMed] [Google Scholar]
- 12.Mlyniec A, Dabrowska S, Heljak M, et al. The dispersion of viscoelastic properties of fascicle bundles within the tendon results from the presence of interfascicular matrix and flow of body fluids. Materials Science and Engineering: C 130 (2021): 112435. [DOI] [PubMed] [Google Scholar]
- 13.Edwards JH, Ingham E, Herbert A. Decellularisation affects the strain rate dependent and dynamic mechanical properties of a xenogeneic tendon intended for anterior cruciate ligament replacement. J Mech Behav Biomed Mater 91 (2019): 18–23. [DOI] [PubMed] [Google Scholar]
- 14.Chandrashekar N, Hashemi J, Slauterbeck J, et al. Low-load behaviour of the patellar tendon graft and its relevance to the biomechanics of the reconstructed knee. Clinical Biomechanics 23 (2008): 918–925. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhury S, Holland C, Thompson MS, et al. Tensile and shear mechanical properties of rotator cuff repair patches. J Shoulder Elbow Surg 21 (2012) 1168–1176. [DOI] [PubMed] [Google Scholar]
- 16.Bozkurt B, Aguilar D, Deswal A, et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 134 (2016): e535–e578. [DOI] [PubMed] [Google Scholar]
- 17.Beason DP, Hsu JE, Marshall SM, et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg 22 (2013): 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couppé C, Hansen P, Kongsgaard M, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107 (2009): 880–886. [DOI] [PubMed] [Google Scholar]
- 19.Halder A, Zobitz ME, Schultz F, et al. Mechanical properties of the posterior rotator cuff. Clinical Biomechanics 15 (2000): 456–462. [DOI] [PubMed] [Google Scholar]
- 20.Thorpe CT, Godinho MSC, Riley GP, et al. The interfascicular matrix enables fascicle sliding and recovery in tendon, and behaves more elastically in energy storing tendons. J Mech Behav Biomed Mater 52 (2015): 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouda MB, Thankam FG, Dilisio MF, et al. Alterations in tendon microenvironment in response to mechanical load: potential molecular targets for treatment strategies. Am J Transl Res 9 (2017): 4341–4360. [PMC free article] [PubMed] [Google Scholar]
- 22.Grant CA, Brockwell DJ, Radford SE, et al. Effects of hydration on the mechanical response of individual collagen fibrils. Appl Phys Lett 92 (2008): 233902. [Google Scholar]
- 23.Lozano PF, Scholze M, Babian C, et al. Water-content related alterations in macro and micro scale tendon biomechanics. Sci Rep 9 (2019): 7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Depalle B, Qin Z, Shefelbine SJ, et al. Influence of cross-link structure, density and mechanical properties in the mesoscale deformation mechanisms of collagen fibrils. J Mech Behav Biomed Mater 52 (2015): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh D, Rai V, Agrawal DK. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol Cardiovasc Med 07 (2023): 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohindra R, Mohindra R, Agrawal DK, et al. Bioactive extracellular matrix fragments in tendon repair. Cell Tissue Res 390 (2022): 131–140. [DOI] [PubMed] [Google Scholar]
- 27.Thankam FG, Diaz C, Chandra I, et al. Hybrid interpenetrating hydrogel network favoring the bidirectional migration of tenocytes for rotator cuff tendon regeneration. J Biomed Mater Res B Appl Biomater 110 (2022): 467–477. [DOI] [PubMed] [Google Scholar]
- 28.Thankam FG, Agrawal DK. Hypoxia-driven secretion of extracellular matrix proteins in the exosomes reflects the asymptomatic pathology of rotator cuff tendinopathies. Can J Physiol Pharmacol 99 (2021): 224–230. [DOI] [PubMed] [Google Scholar]
- 29.Fang W, Sekhon S, Teramoto D, et al. Pathological alterations in the expression status of rotator cuff tendon matrix components in hyperlipidemia. Mol Cell Biochem 478 (2023): 1887–1898. [DOI] [PubMed] [Google Scholar]
- 30.Fang WH, Bonavida V, Agrawal DK, et al. Hyperlipidemia in tendon injury: chronicles of low-density lipoproteins. Cell Tissue Res 392 (2023): 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rai V, Dietz NE, Dilisio MF, et al. Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res Ther 18 (2016): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 26 (2017): 2086–2090. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y-K, Jung K-H, Kim J-W, et al. Factors affecting rotator cuff integrity after arthroscopic repair for medium-sized or larger cuff tears: a retrospective cohort study. J Shoulder Elbow Surg 27 (2018): 1012–1020. [DOI] [PubMed] [Google Scholar]


