Fig. 5.
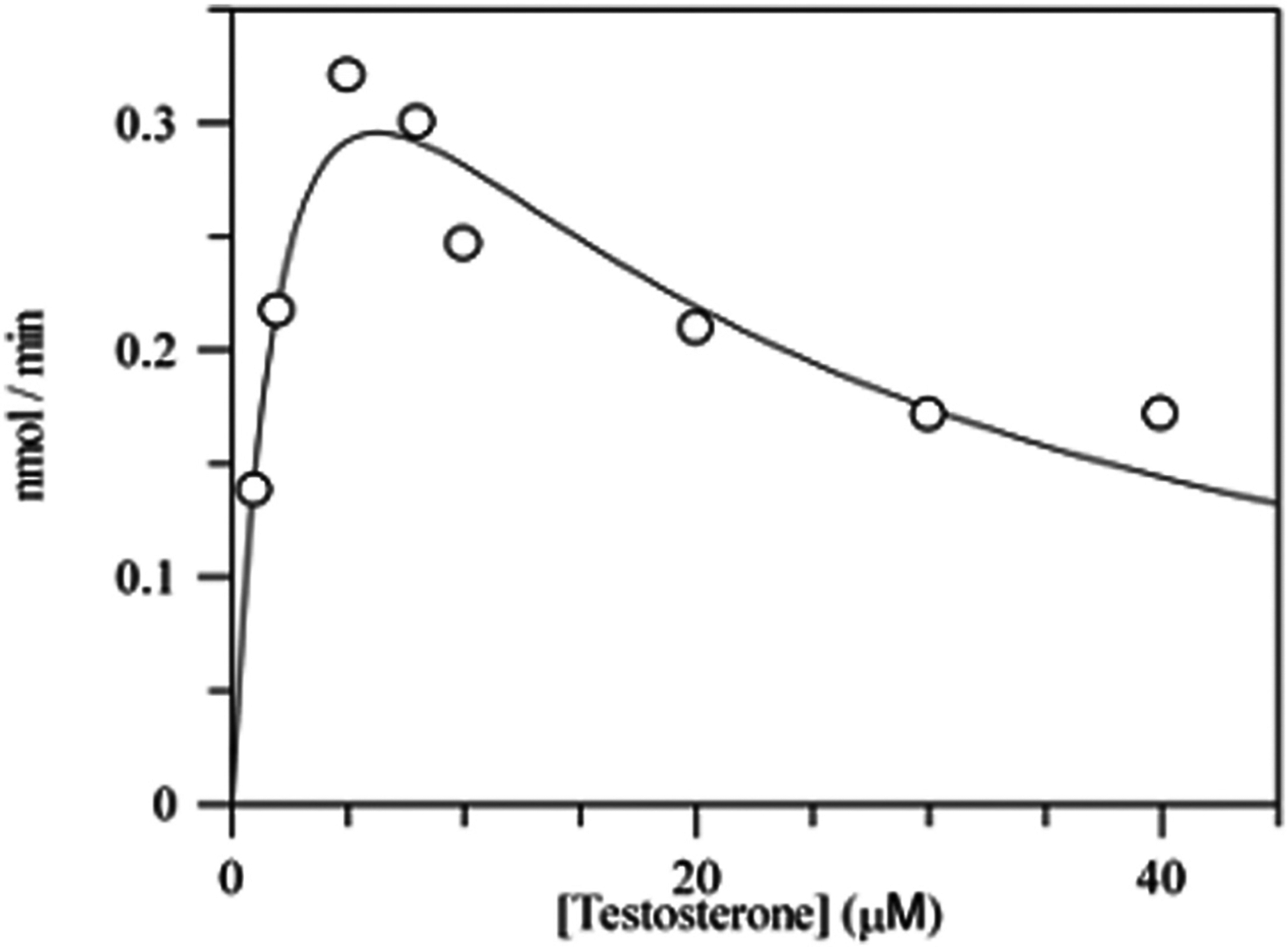
Substrate inhibition of AKR1D1 by testosterone. Velocity versus substrate plot for AKR1D1 showing substrate inhibition using testosterone. Assays contained 0.078 μM enzyme, 1–40 μM testosterone, 12 μM NADPH, 4% acetonitrile in 100 mM potassium phosphate buffer, pH 6.0, in a final volume of 1 mL. Reactions were monitored fluorometrically by using an excitation wavelength of 340 nm (slit-width 5 nm) and an emission wavelength set at 450 nm (slit-width 10 nm) at 37 °C. Kinetic analysis of initial velocities obtained was performed using the Henri-Michaelis-Menten equation for uncompetitive substrate inhibition v = (Vmax × [S])/(Km + [S] + [S]2/Ki) and fit using the program GraphFit. The iterative fits provided estimates of Vmax, Km and Ki and associated standard errors. This research was originally published in the Journal of Biological Chemistry. Di Costanzo et al., (2008).
