Abstract
BACKGROUND:
Anthracycline-induced cardiotoxicity has a variable incidence, and the development of left ventricular dysfunction is preceded by elevations in cardiac troponin concentrations. Beta-adrenergic receptor blocker and renin-angiotensin system inhibitor therapies have been associated with modest cardioprotective effects in unselected patients receiving anthracycline chemotherapy.
METHODS:
In a multicenter, prospective, randomized, open-label, blinded end-point trial, patients with breast cancer and non-Hodgkin lymphoma receiving anthracycline chemotherapy underwent serial high-sensitivity cardiac troponin testing and cardiac magnetic resonance imaging before and 6 months after anthracycline treatment. Patients at high risk of cardiotoxicity (cardiac troponin I concentrations in the upper tertile during chemotherapy) were randomized to standard care plus cardioprotection (combination carvedilol and candesartan therapy) or standard care alone. The primary outcome was adjusted change in left ventricular ejection fraction at 6 months. In low-risk nonrandomized patients with cardiac troponin I concentrations in the lower 2 tertiles, we hypothesized the absence of a 6-month change in left ventricular ejection fraction and tested for equivalence of ±2%.
RESULTS:
Between October 2017 and June 2021, 175 patients (mean age, 53 years; 87% female; 71% with breast cancer) were recruited. Patients randomized to cardioprotection (n=29) or standard care (n=28) had left ventricular ejection fractions of 69.4±7.4% and 69.1±6.1% at baseline and 65.7±6.6% and 64.9±5.9% 6 months after completion of chemotherapy, respectively. After adjustment for age, pretreatment left ventricular ejection fraction, and planned anthracycline dose, the estimated mean difference in 6-month left ventricular ejection fraction between the cardioprotection and standard care groups was −0.37% (95% CI, −3.59% to 2.85%; P=0.82). In low-risk nonrandomized patients, baseline and 6-month left ventricular ejection fractions were 69.3±5.7% and 66.4±6.3%, respectively: estimated mean difference, 2.87% (95% CI, 1.63%–4.10%; P=0.92, not equivalent).
CONCLUSIONS:
Combination candesartan and carvedilol therapy had no demonstrable cardioprotective effect in patients receiving anthracycline-based chemotherapy with high-risk on-treatment cardiac troponin I concentrations. Low-risk nonrandomized patients had similar declines in left ventricular ejection fraction, bringing into question the utility of routine cardiac troponin monitoring. Furthermore, the modest declines in left ventricular ejection fraction suggest that the value and clinical impact of early cardioprotection therapy need to be better defined in patients receiving high-dose anthracycline.
REGISTRATION:
URL: https://doi.org; Unique identifier: 10.1186/ISRCTN24439460. URL: https://www.clinicaltrialsregister.eu/ctr-search/search; Unique identifier: 2017-000896-99.
Keywords: adrenergic beta-antagonists; angiotensin receptor antagonists; breast neoplasms; cardiomyopathies; lymphoma, non-Hodgkin; magnetic resonance imaging; troponin
Clinical Perspective.
What Is New?
In this randomized controlled trial of patients at high risk for anthracycline cardiotoxicity, combined candesartan and carvedilol therapy did not protect against decline in 6-month left ventricular ejection fraction after completion of chemotherapy.
Overall decline in 6-month left ventricular ejection fraction occurred regardless of changes in cardiac troponin concentration during chemotherapy.
What Are the Clinical Implications?
The Cardiac CARE trial (High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy) findings do not support recent guideline recommendations advocating the use of cardiac troponin monitoring and early preventive neurohormonal blockade in patients at risk of anthracycline cardiotoxicity.
Future studies should focus on factors determining transition to subsequent development of heart failure from initial mild and asymptomatic changes in cardiac function after anthracycline chemotherapy.
Anthracyclines are effective cytotoxic drugs that contribute to improved survival in a wide range of cancers, including breast cancer and lymphoma. Anthracyclines cause dose-related cardiomyocyte injury, leading to left ventricular dysfunction and heart failure.1 Improved cancer-free survival has led to growing concern about the impact of cancer therapy–related cardiac dysfunction (CTRCD).2,3 However, progression from heart muscle injury at the time of chemotherapy to the development of clinical heart failure is poorly understood, and the overall utility of potentially cardioprotective treatments has not been established.4 Although extremes of age, preexisting cardiovascular disease, and cumulative anthracycline dose are risk markers for CTRCD,5 most anthracycline-treated patients do not develop clinically important cardiotoxicity.6 The degree of CTRCD observed in recent cardioprotection trials has been lower than in historical studies; this may reflect a trend for the use of lower-dose anthracycline regimens in high-risk patients. Indeed, Cardinale’s group reported clinical heart failure in 7% and asymptomatic left ventricular dysfunction in an additional 5% of 703 patients 1 year after receiving anthracycline containing high-dose chemotherapy.7 In contrast, 2 recent studies following up 399 randomized patients for at least 2 years reported only one episode (0.2%) of clinical heart failure.8,9
International guidelines recommend cardiac troponin monitoring and cardiac imaging during and after anthracycline treatment in patients at risk of CTRCD.5 The use of potentially cardioprotective neurohormonal antagonists has been advocated for patients who are at increased risk of CTRCD because of clinical factors such as exposure to high cumulative anthracycline doses, as well as patients who develop biomarker or cardiac imaging evidence of CTRCD. Cardiac troponin has been used to detect early anthracycline-induced cardiomyocyte toxicity.7,10 Our group and others have shown that plasma high-sensitivity cardiac troponin concentrations below the 99th centile upper reference limit provide prognostic information and identify individuals with and without cardiac symptoms who are at heightened risk of cardiac events and mortality.11,12 Furthermore, early changes below the 99th centile upper reference limit during anthracycline treatment identify those who go on to develop myocardial injury soon after chemotherapy.13
Set against the background of apparently declining event rates and a minority of patients developing CTRCD, previous clinical trials have adopted an unselected approach to randomization. With the inclusion of patients at low risk of CTRCD, the potential to demonstrate substantial cardioprotective effects of neurohormonal blockade has been challenging.8,14 In addition, most trials have used treatments that block either the renin-angiotensin system (angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist) or the sympathetic nervous system (β-adrenoreceptor antagonist) but not the combination, which has the most robust evidence base for improving function and survival in patients with left ventricular systolic dysfunction. The PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) investigated whether anthracycline-treated patients with breast cancer were protected from CTRCD with candesartan or metoprolol.8 The overall decline in left ventricular ejection fraction (LVEF) on cardiac magnetic resonance was only 2.6 percentage points in the placebo group. Candesartan but not metoprolol had an early protective effect on LVEF decline that was not maintained on extended follow-up.15 Consequently, it has been suggested that future trials examining cardioprotective therapy should preferentially be conducted in patients at high risk of CTRCD.16
The objectives of the Cardiac CARE trial (High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy) were to determine whether cardiac troponin monitoring identifies patients at risk of left ventricular systolic dysfunction during anthracycline chemotherapy and whether cardiac troponin–guided treatment with candesartan and carvedilol prevents the development of left ventricular systolic dysfunction. These objectives have immediate relevance to clinical practice and current guideline recommendations through testing a simple monitoring and threshold-guided intervention pathway that can readily be delivered within cancer treatment centers.
METHODS
Study Design and Participants
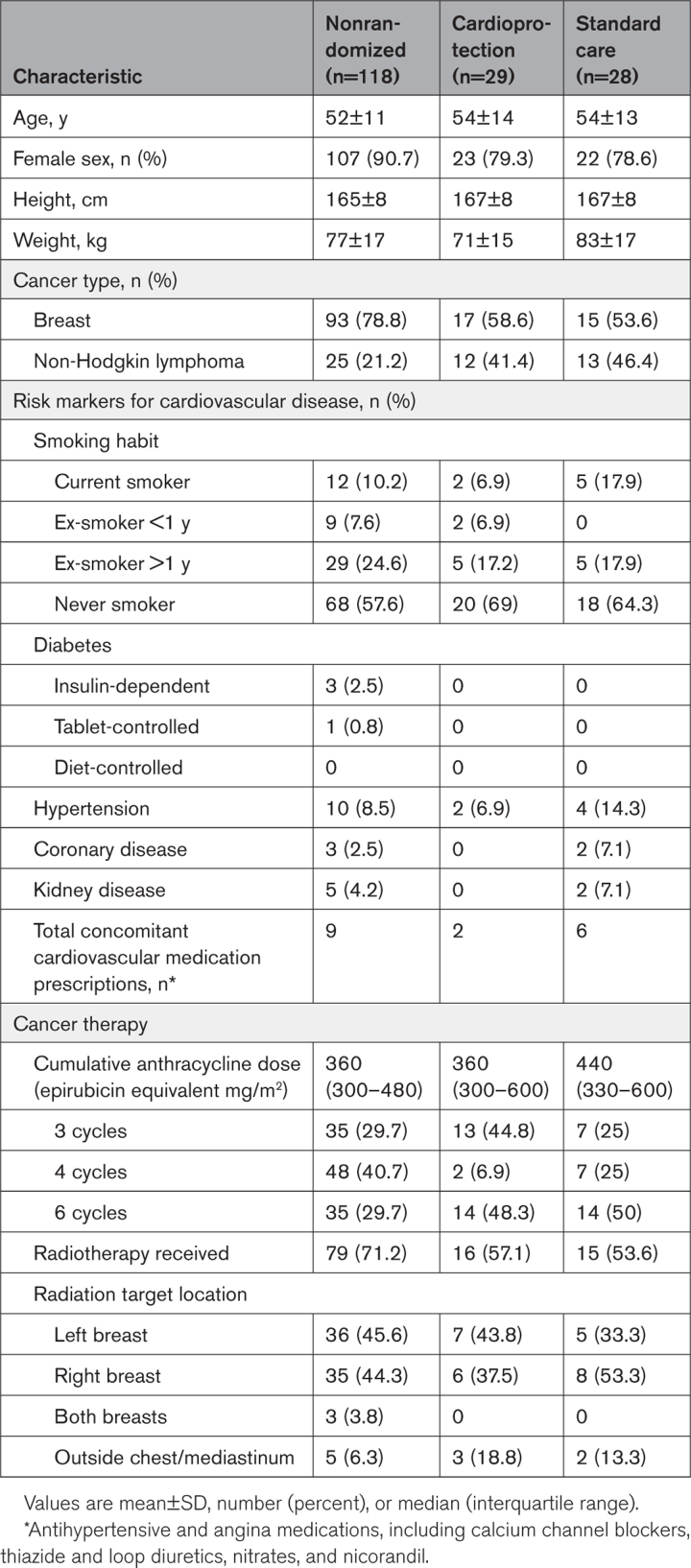
The data that support the findings of this study are available from the corresponding author on reasonable request. This was a multicenter, prospective, randomized, open-label, blinded end-point trial nested within an observational cohort study. Study methods and design have been described previously.17 Eligible patients were women and men >18 years of age with LVEF ≥50% on baseline cardiac magnetic resonance imaging and without serious comorbidity who were scheduled for anthracycline-containing therapy for breast cancer or non-Hodgkin lymphoma. Key exclusion criteria were human epidermal growth factor receptor 2–positive breast cancer (those patients would be scheduled for anti–human epidermal growth factor receptor 2 therapy); lower-dose anthracycline regimens (cumulative epirubicin equivalent dose <300 mg/m2); ongoing treatment with angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, or beta-blockers; hypotension or hypertension; or previous anthracycline chemotherapy (Table 1). All trial participants provided written informed consent before study procedures were conducted. The study was conducted in accordance with the Declaration of Helsinki and approved by the South-East Scotland Research Ethics Committee (17/ES/0071).
Table 1.
Baseline Characteristics of the 3 Study Groups
Study Procedures
Cardiac Magnetic Resonance
Cardiac magnetic resonance scans were conducted with steady-state free-precession breath-hold cines on 1.5-T and 3-T magnetic resonance imaging scanners and were performed at baseline before chemotherapy was started and 6 months after the final anthracycline dose. Cardiac magnetic resonance scan results were made immediately available at each site to inform ongoing clinical care. Cardiac magnetic resonance measurements for the primary and secondary outcomes, including LVEF, global longitudinal and circumferential strain with cardiac magnetic resonance feature tracking, left ventricular volume and mass, and left atrial area, were performed by 2 analysts in the Core Image Analysis laboratory (Edinburgh Imaging, University of Edinburgh) according to the Society for Cardiovascular Magnetic Resonance guidelines on dedicated software (CVI42 version 5.14, Circle Cardiovascular Imaging). Both analysts were independent of the research teams and blinded to scan sequence (before or after chemotherapy) and treatment allocation.
Cardiac Troponin Monitoring
Cardiac troponin I concentrations were quantified with the ARCHITECTSTAT or ALINITY cardiac troponin assay (Abbott Laboratories, Chicago, IL) during each 3-week chemotherapy cycle. This assay has an interassay coefficient of variation of <10% at 4.7 ng/L, a limit of detection of 1.9 ng/L, and a 99th centile upper reference limit of 34 ng/L in men and 16 ng/L in women.18 Patients had cardiac troponin concentrations measured before randomization; before each cycle of anthracycline chemotherapy; and 2, 4, and 6 months after completion of chemotherapy.
Study Randomization and Intervention
After enrollment and a baseline cardiac magnetic resonance scan, cardiac troponin concentrations were measured before each cycle of anthracycline. Patients could be randomized from cycle 2 to cycle 6. Treatment allocation was by dynamic 1:1 randomization with minimization for prognostic factors: (1) age ≥65 or <65 years; (2) baseline LVEF ≥60% or <60%; and (3) planned cumulative epirubicin equivalent dose 300 or >300 mg/m2. Cardiac troponin concentration thresholds that triggered randomization were ≥5 ng/L for cycle 2 and ≥23 ng/L for cycles 3 to 6. These thresholds were defined in a previous study as identifying patients most likely to develop myocardial injury on completion of the course of chemotherapy.13 Nonrandomized participants were treated with standard care.
The trial intervention consisted of candesartan started at 8 mg daily and increased to 16 and 32 mg daily, and carvedilol was started at 6.25 mg twice daily and was increased to 12.5 and 25 mg twice daily. Combination candesartan and carvedilol therapy was dispensed within 14 days of randomization and continued until completion or withdrawal from the study. Dose restrictions and modifications were performed according to blood pressure, heart rate, and renal function.
Outcomes
The primary efficacy outcome was adjusted change in LVEF from baseline to 6 months after the final anthracycline dose determined by cardiac magnetic resonance imaging and compared between the randomized groups. The trial also aimed to determine whether early changes in cardiac troponin concentration on anthracycline treatment identified patients at low and high risk of cardiotoxicity. To evaluate the effectiveness of cardiac troponin monitoring, the key secondary efficacy outcome was absence of change in LVEF in the low-risk nonrandomized group with equivalence limits of ±2%.19
Other secondary efficacy outcomes included change in global longitudinal and circumferential strain, left ventricular mass and volume, and left atria area determined by cardiac magnetic resonance imaging, as well as change in cardiac troponin concentration. We have previously outlined the reporting plan for clinical outcomes, including cardiovascular death; new-onset heart failure; additional cardiac magnetic resonance and cardiac troponin definitions of asymptomatic CTRCD; and heart rate, blood pressure and safety outcomes.17 The analysis plan included additional exploratory comparisons across cardiotoxicity measures between low-risk nonrandomized and high-risk randomized participants. These were performed to compare the magnitude of plasma cardiac troponin concentration increase in randomized and nonrandomized participants and to determine how closely the trial protocol identified a population at risk of cardiotoxicity.
The following investigational medicinal product safety outcomes were compared between treatment groups: hypotension (systolic blood pressure <90 mm Hg), bradycardia (heart rate <50 bpm), hyperkalemia (≥5.0 mmol/L), worsening renal function, acute kidney injury, fatigue, and new atrial fibrillation.
Sample Size
We planned to randomize at least 33% of participants using the previously defined cardiac troponin thresholds.17 We assumed that this threshold would select all participants developing clinically important reductions in LVEF. Assuming an SD of 5% in LVEF,20 we required 23 participants per group to detect a 5% change in LVEF at 90% power and 2-sided P<0.05. This effect size was used in the PRADA study.20 Allowing for 1 in 6 participants with missing data, the total randomized trial sample size was 56. Assuming that one-third of participants would be randomized into the trial, the total observational study cohort size was at least 168 participants.
To assess the effectiveness of cardiac troponin monitoring, we measured change in LVEF in the low-risk nonrandomized group, defining an absence of change in LVEF as being within ±2% of the baseline measurement. At 90% power and 2-sided P<0.05 and assuming a similar SD of 5%, paired cardiac magnetic resonance imaging scans would be required in 68 nonrandomized participants.
Statistical Analysis
Analyses were performed with participants in their randomized groups, regardless of adherence, apart from adverse events; for adverse events, participants were analyzed by treatment received. Descriptive data are presented as number (percentage), mean±SD, or median (lower and upper quartiles). Analyses tested for differences unless specified. Statistical tests for a difference were 2 sided with 5% significance level and 95% CIs. There was no adjustment for multiplicity. In each analysis, participants with missing outcome data were omitted (complete case analysis). When analyses were adjusted, binary fixed effects for the minimization variables were added into the model: age ≥65 or <65 years, baseline LVEF ≥60% or <60%, and planned cumulative epirubicin equivalent dose 300 or >300 mg/m2. All analyses were carried out with SAS version 9.4. A full statistical analysis plan was finalized before database lock.
The primary outcome of LVEF change from baseline to 6 months after completion of chemotherapy was compared between the randomized intervention groups using linear regression, adjusted as specified previously, and presented using the between-intervention group difference in means. An unadjusted analysis was presented as a sensitivity analysis. There were no preplanned subgroup analyses. The same method was used for other cardiac imaging measures for change in high-sensitivity cardiac troponin I from baseline to 2 months after chemotherapy and as a post hoc analysis for heart rate and blood pressure.
To assess the specificity of high-sensitivity cardiac troponin I for cardiotoxicity on LVEF, within the nonrandomized group, 6-month posttreatment LVEF and baseline LVEF were compared. The aim was to demonstrate equivalence. We calculated the mean of the within-person changes in LVEF plus its 95% CI, which was compared with the equivalence limits of ±2%, using two 1-sided tests.
We presented the mean of the within-person changes between participants’ magnetic resonance imaging scans before and after anthracycline for the group randomized to standard care (high-risk) and for the nonrandomized group (low-risk). We calculated P values using linear regression, adjusted as specified previously. The same method was used for other cardiac magnetic resonance measures.
The area under the curve of all study high-sensitivity cardiac troponin I measurements taken between baseline and the final cycle of chemotherapy was calculated for each participant using the trapezium rule. To be included, participants had to have a baseline value and ≥4 values in total. We presented summaries separately by number of chemotherapy cycles for the nonrandomized group and the randomized standard care group and compared these 2 groups using t tests.
We initially planned to perform formal survival analysis for death, cardiovascular disease, and heart failure and logistic regression analyses for hypotension, bradycardia, hyperkalemia, worsening renal function, acute kidney injury, new diagnosis of atrial fibrillation, and fatigue, but these analyses were not performed due to the low numbers of events.
RESULTS
Study Population
Between October 4, 2017, and June 30, 2021, 424 patients were approached across 7 centers in the United Kingdom (Figure 1). Of these, 191 patients (45.0%) consented to participation, and 16 patients were excluded. From 175 patients included in the observational cohort, 57 (32.6%) were randomized into the trial: 50 (87.7%) were randomized at anthracycline treatment cycle 2, 4 (7.0%) at cycle 5, and 3 (5.3%) at cycle 6.
Figure 1.
Cardiac CARE Consolidated Standards of Reporting Trials diagram. Cardiac CARE indicates High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy; FU, follow-up; MRI, magnetic resonance imaging; and PIL, patient information leaflet.
The study population was composed predominantly of women with breast cancer (Table 1). Patients with non-Hodgkin lymphoma were proportionately more likely to be randomized than patients with breast cancer. Cardiovascular risk factors and concomitant cardiovascular medications were uncommon across all groups. Mean anthracycline dose was higher in the randomized groups. Radiotherapy was more commonly prescribed in the nonrandomized group (71.2%) compared with the cardioprotection (57.1%) and standard care (53.6%) groups.
Compliance With Treatment Allocation
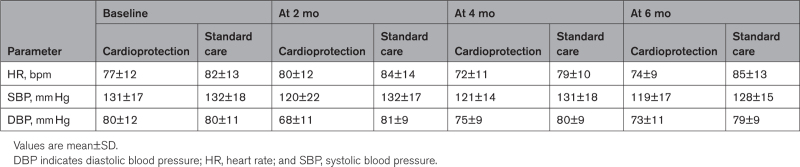
Twenty patients (68.9%) were adherent to cardioprotection treatment at 6 months, although one patient stopped candesartan within 2 months and continued with carvedilol alone. Two patients (6.9%) randomized to cardioprotection did not receive any medication because of intercurrent illness and COVID-19 infection. An additional 7 participants (24.1%) stopped both cardioprotection drugs within 2 months due tosymptoms of light-headedness and dizziness possibly related to low blood pressure. Blood pressure and heart rate were lower in the cardioprotection treatment group at 6 months (Table 2). On post hoc analysis, there was a greater reduction in heart rate at 6 months in the cardioprotection group (estimated mean difference, –11 bpm [95% CI, −18 to −4]; P=0.003). Blood pressure appeared to be lower in the cardioprotection group, but changes in systolic (−7 mm Hg [95% CI, −17 to 2.0]; P=0.12) and diastolic (−6 mm Hg [95% CI, −13 to 0.2]; P=0.06) pressures were not statistically significant.
Table 2.
Participant Hemodynamic Measures at Baseline and 2, 4, and 6 Months After Completion of Chemotherapy
Primary and Key Secondary Outcomes
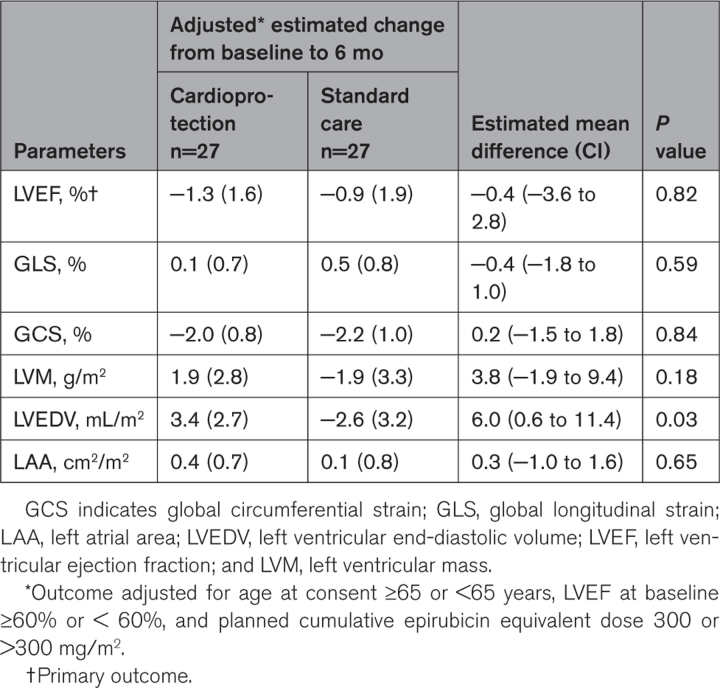
Patients randomized to cardioprotection or standard care had LVEFs of 69.4±7.4% and 69.1±6.1% at baseline and 65.7±6.6% and 64.9±5.9% 6 months after completion of chemotherapy, respectively (Table 3). After adjustment for age, pretreatment LVEF, and planned anthracycline dose, there was no change in the estimated mean difference in 6-month LVEF between the cardioprotection and standard care groups (−0.37 percentage points [95% CI, −3.59 to 2.85]; P=0.82; Table 4; Figure 2).
Table 3.
Participant Cardiac MRI Measures at Baseline and 6 Months After Anthracycline
Table 4.
Adjusted Change in Cardiac Magnetic Resonance Measures From Baseline to 6 Months After Final Anthracycline Dose
Figure 2.
Central illustration. Primary outcome: change in left ventricular ejection fraction (LVEF).
In nonrandomized patients, the baseline and 6-month LVEFs were 69.3±5.7% and 66.4±6.3%, respectively. The estimated nonadjusted mean difference was 2.87%, and its 95% CI was 1.63% to 4.10%. Hence, the main secondary objective of demonstrating no change in LVEF with equivalence of ±2% was not met (P=0.92).
In post hoc sensitivity analyses, we assessed the per-protocol primary efficacy outcome between randomized groups. There was no difference in outcome when only the 19 cardioprotection patients who were fully adherent to treatment allocation were included. The estimated mean difference in the change in 6-month LVEF between the cardioprotection and standard care groups was −0.7 percentage points (95% CI, −4.3 to 2.9; P=0.70).
Secondary Outcomes
Cardiac troponin I concentrations increased progressively in all groups during chemotherapy (Table S1). By design, cardiac troponin concentrations were higher in the randomized groups (Figure S1; Table S2). Adjusted estimated mean change (±standard error) in cardiac troponin concentration from baseline to 2 months after chemotherapy in the cardioprotection and standard care groups was 27.3±7.4 and 28.8±8.8 ng/L, respectively. The adjusted estimated mean difference was −1.55 ng/L (95% CI, −17.56 to 14.45; P=0.85). A difference between the cardioprotection and standard care groups was observed for the secondary outcome of adjusted left ventricular end-diastolic volume indexed for body surface area (Table 4). However, there were no differences for global longitudinal and circumferential strain, left ventricular mass, and left atrial area.
Exploratory comparisons were conducted between the high-risk standard care group and low-risk nonrandomized group. The nonadjusted mean changes in LVEF were similar in the low-risk nonrandomized participants (−2.9±6.1%) and high-risk standard care group (−4.33±4.4%; mean difference, −1.46% [95% CI, −3.96% to 1.03%]; P=0.25). The adjusted estimated mean difference in LVEF decline between these 2 groups was also similar. Adjusted estimated mean differences in all cardiac magnetic resonance measures of cardiotoxicity between the high-risk standard care group and low-risk nonrandomized groups were again similar (Table S3).
Clinical Outcomes
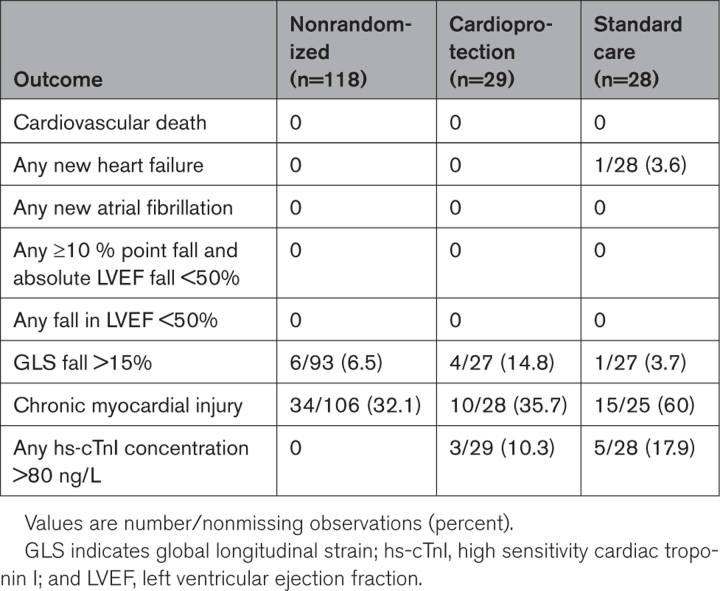
No cardiovascular deaths or incident episodes of atrial fibrillation were recorded during the trial (Table 5). One patient randomized to the standard care group developed heart failure and received treatment, including candesartan. This patient’s LVEF recovered on the 6-month cardiac magnetic resonance scan. No patients met the CTRCD criterion of a 10-percentage-point LVEF decrease or a decrease to an absolute LVEF <50%. Similarly, the CTRCD criterion of a >15% fall in global longitudinal strain was uncommon across groups. Chronic myocardial injury, defined as an elevated cardiac troponin concentration above the 99th centile upper reference limit 2 months after completion of chemotherapy, was common and similar in the nonrandomized (32.1%) and cardioprotection (35.7%) groups. The proportion with chronic myocardial injury was higher (60%) in the standard care treatment group. Recordings of high (>80 ng/L) cardiac troponin concentrations were confined to the randomized groups.
Table 5.
Participant Clinical Outcomes and Measures of Chemotherapy-Related Cardiac Dysfunction
Safety Outcomes
Adverse events were more commonly reported in the cardioprotection group, with 71.4% of patients having at least one adverse event compared with 10.3% standard-care patients and 12.7% nonrandomized patients (Table 6). During the trial, there were 25 reportable serious adverse events, with 12 in the cardioprotection group compared with 2 in the standard care group. Most adverse events in the cardioprotection group were possibly related to the investigational medicinal products, with dizziness and syncope listed in 17 of 20 possibly related adverse events and hypotension, palpitation, and venous thromboembolism listed for the remaining adverse events, with possible causal links to the investigational medicinal product. In contrast to adverse event reporting, there was no signal of harm related to investigational medicinal product prescription in safety reporting. There were no patients with protocol-defined hypotension or bradycardia at study visits after completion of chemotherapy. Hyperkalemia at any point after randomization occurred in 10.3% of nonrandomized patients and was more common in the cardioprotection (20.7%) and standard-care (17.9%) groups. Worsening renal function at any point beyond baseline occurred in 2.7%, 6.9%, and 7.1% of the nonrandomized, cardioprotection, and standard-care groups, respectively. Fatigue was reported by 12.1%, 3.4%, and 25.0% of the nonrandomized, cardioprotection, and standard-care groups, respectively.
Table 6.
Patient Adverse Event Reporting
DISCUSSION
We found no strong evidence that early cardioprotection therapy with combined candesartan and carvedilol therapy prevented 6-month decline in LVEF in patients with breast cancer or non-Hodgkin lymphoma. This was despite enrichment by randomizing only patients with high cardiac troponin concentrations and clear evidence of pharmacological effect, with changes in heart rate and left ventricular end-diastolic volume in the cardioprotection group. Moreover, LVEF decline was similar in low-risk nonrandomized and high-risk randomized patient groups despite substantial differences in cardiac troponin concentrations during anthracycline treatment. Overall, our findings bring into question the benefit of guidelines that advocate treatment based on cardiac troponin monitoring to identify patients at risk of anthracycline CTRCD and early intervention with cardioprotection therapy in patients with the highest levels of cardiac troponin.
Key strengths of this multicenter trial include cardiac magnetic resonance imaging with core laboratory quantification and use of the same high-sensitivity cardiac troponin I assay across all sites to provide precise and standardized measures of myocardial injury. Recruitment and randomization goals were exceeded, and data completeness for the primary and main secondary outcomes was excellent despite the challenge of the COVID-19 pandemic that interrupted trial recruitment for 6 months.
The decline in LVEF at 6 months was smaller than in previous studies using echocardiographic monitoring21,22 but comparable to that in recent multicenter studies enrolling similar patient populations using cardiac magnetic resonance.8,9 We observed small deteriorations in both global and longitudinal strain across all groups. There was no difference in these early markers of ventricular dysfunction between randomized groups, and the cardiotoxicity threshold of >15% relative fall in strain was uncommon at 6 months. The increase in left ventricular end-diastolic volume in the cardioprotection group was likely related to the impact of beta-blockers slowing heart rate with consequent increased filling and stroke volume.
Candesartan and carvedilol therapy did not reduce cardiac troponin concentration change from baseline to 2 months after chemotherapy. We identified this as a time point to examine for a treatment effect when cardiac troponin concentrations might be expected to remain elevated after completion of chemotherapy and when patients randomized to cardioprotection would have received therapy for at least 2 months. Participants with the highest concentrations of cardiac troponin (any measurement >80 ng/L) were confined to randomized groups, and chronic myocardial injury, defined as a persistent elevation in cardiac troponin above the 99th centile upper reference limit, 2 months after chemotherapy was common across all 3 groups. We believe this is the first time that this persistent signal of myocardial injury, present in 60% of those randomized to the standard-care group, has been demonstrated in a large population of patients with anthracycline cardiotoxicity.
Combined therapy with candesartan and carvedilol initiated during chemotherapy was associated with adverse effects, and 31% of patients stopped or did not start cardioprotection therapy within 2 months of randomization. Symptoms possibly related to cardioprotection medication, such as dizziness, were frequently listed in adverse event reporting as the reason for early cessation. In contrast, the rate of nonadherence was lower in the PRADA study, with 7% of patients assigned to the combined metoprolol and candesartan therapy arm discontinuing medication.8 This may reflect use of a placebo control in this study. As in PRADA, we found no evidence for a signal of excess harm related to cardioprotection therapy in the protocol-specified investigational medicinal product safety outcomes. Fatigue was more common in those randomized to standard care. This result was unexpected, given that fatigue is a side effect commonly attributed to beta-blocker use.
Limitations
Several patients discontinued cardioprotection medication within 2 months of randomization, and this may have had some influence on treatment effect. The trial was powered to detect a 5-percentage-point difference in LVEF between randomized groups, and there was no evidence of greater or less LVEF decline with cardioprotection. However, we cannot exclude a small treatment effect, although the upper boundary of the 95% CI for the primary end point was 2.85%. Finally, by design, there was a predominance of women, making up 79% of randomized patients. This may limit the applicability of the results to men.
Conclusions
Six months after completion of anthracycline chemotherapy, combined candesartan and carvedilol did not prevent a small decline in LVEF or impact other secondary CTRCD measures. Our results are in accordance with recent trials investigating neurohormonal blockade in anthracycline-treated patients.14,15
The trial protocol successfully identified participants who developed high on-treatment cardiac troponin concentrations for randomization, but despite being considered high risk, the degree of CTRCD in the randomized group was mild and not demonstrably different from that of low-risk nonrandomized participants. Cardiac troponin I appears to be an excellent marker of anthracycline myocardial injury and has been advocated in the 2022 European Society of Cardiology cardio-oncology clinical guidelines to detect anthracycline CTRCD and to guide cardioprotection therapy.5 The Cardiac CARE results underline the importance of testing surrogate markers of CTRCD in the randomized controlled trial setting before adopting them into clinical practice.
Cardioprotection therapy was poorly tolerated by some participants, leading to drug-related side effects and discontinuations. The small decline in LVEF observed in Cardiac CARE will not have immediate clinical implications for individual patients. Applied across a population, this LVEF decline may confer a general increased risk of future cardiac dysfunction and heart failure. Our findings show that the benefit of targeted combined cardioprotection therapy with candesartan and carvedilol is uncertain, and the treatment was not well tolerated. Future research should be directed at understanding factors determining evolution of late cardiac dysfunction in this patient population with more prolonged monitoring, long-term imaging, and clinical follow-up.
ARTICLE INFORMATION
Acknowledgments
The authors thank all the patients, site staff, image analysis team, the trial steering committee, data monitoring committee, and patient advisory group. They also thank the Edinburgh clinical trials unit staff for their involvement. The University of Edinburgh and the Lothian Health Board are cosponsors. The authors thank the National Institute of Health Research (NIHR) and British Heart Foundation for funding the trial and for their constant support and advice. They acknowledge the support of the NIHR clinical research network. Magnetic resonance imaging analysis was expertly performed at the Edinburgh imaging facility, University of Edinburgh. The following groups contributed substantially to the trial. The authors gratefully acknowledge the BHF Cardiovascular Biomarker Laboratory, University of Edinburgh, for their assistance with this work. Trial steering committee: Stephen Evans (London School of Hygiene and Tropical Medicine, UK), Torbjørn Omland (Akershus, Oslo, Norway), Abigail Marks (patient representative), and Dr Gordon Urquhart (consultant medical oncologist at Aberdeen Royal Infirmary, UK). data monitoring committee: Helena Earl (chair before retirement, consultant medical oncologist at Addenbrooke’s Hospital, UK), Dr Mark Francis (chair, consultant cardiologist at NHS Fife, UK), Dr Ellen Copson (University of Southampton, UK), Dr Dominic Culligan (consultant hematologist at Aberdeen Royal Infirmary and the University of Aberdeen, UK), and Helen Mossop (Biostatistics Research Group within the Population Health Sciences Institute at Newcastle University, UK). Trial management group: Edinburgh clinical trials unit staff: Anna Foster (trial management support), Hannah Rickman (trial management support), Garry Milne (database programmer), Lynsey Milne (data management), and Linda Williams (unmasked statistician). Edinburgh Imaging Facility: Scott Semple (professor of medical imaging and physics) and Annette Cooper (lead radiographer). Image analysis core laboratory: Dr Tom MacGillivray (core laboratory manager and senior research fellow). P.A.H., P.H., O.O., M.M., S.L., D.E.N., N.L.M., and N.L. conceived the study and its design. A.R had access to the data and performed the analysis. P.A.H., S.L., A.R., P.H., O.O., D.E.N., N.L.M., and N.L. interpreted the data and drafted the manuscript. All authors revised the manuscript critically for important intellectual content and provided their final approval of the version to be published. All authors are accountable for the work.
Sources of Funding
Dr Henriksen acknowledges the financial support of National Health Service Research Scotland, through National Health Service Lothian. Dr Henriksen is chief investigator for the Cardiac CARE Study (EudraCT 2017-000896-99, ISRCTN24439460), which is funded by the Efficacy and Mechanism Evaluation Programme (funding reference 15/48/20), a Medical Research Council and NIHR partnership, and the British Heart Foundation. Dr Mills is supported by the British Heart Foundation through a chair award (CH/F/21/90010), program grant (RG/20/10/34966), and research excellent award (RE/18/5/34216). The funders had no role in the study or the decision to submit this work to be considered for publication. Dr Lang is supported by a British Heart Foundation Centre of Research excellence award (RE/18/6/34217). The views expressed in this publication are those of the authors and not necessarily those of the MRC, National Health Service, NIHR, or Department of Health. The Efficacy and Mechanism Evaluation Programme is funded by MRC and NIHR, with contributions from CSO in Scotland and NISCHR in Wales, and the HSC R&D Division, public health agency, in Northern Ireland.
Disclosures
Dr Mills has received personal fees from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, and LumiraDx and has received grants awarded to the University of Edinburgh from Abbott Diagnostics and Siemens Healthineers outside the submitted work. Dr Lang has received personal fees from Akero, Roche, Pfizer, and Novartis and research grant support from Roche Diagnostics outside the submitted work. Dr Williams has given talks at meetings sponsored by Siemens Healthineers, Canon Medical Systems, and Novartis. The other authors report no conflicts.
Supplemental Material
Figure S1
Tables S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- Cardiac CARE
- High-Sensitivity Cardiac Troponin I–Guided Combination Angiotensin Receptor Blockade and Beta Blocker Therapy to Prevent Cardiac Toxicity in Cancer Patients Receiving Anthracycline Chemotherapy
- CTRCD
- cancer therapy–related cardiac dysfunction
- LVEF
- left ventricular ejection fraction
- PRADA
- Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy trial
This manuscript was sent to Ileana L. Piña, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.064274.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 1689.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Peter Hall, Email: p.s.hall@ed.ac.uk.
Iain R. MacPherson, Email: iain.macpherson@glasgow.ac.uk.
Shruti S. Joshi, Email: sjoshi@ed.ac.uk.
Trisha Singh, Email: tsingh@ed.ac.uk.
Morag Maclean, Email: morag.maclean@ed.ac.uk.
Steff Lewis, Email: steff.lewis@ed.ac.uk.
Aryelly Rodriguez, Email: aryelly.rodriguez@ed.ac.uk.
Alex Fletcher, Email: alexander.fletcher.2@glasgow.ac.uk.
Russell J. Everett, Email: russell.ev@gmail.com.
Harriet Stavert, Email: harriet.stavert@nhslothian.scot.nhs.uk.
Angus Broom, Email: angus.broom@nhslothian.scot.nhs.uk.
Lois Eddie, Email: lois.eddie@nhslothian.scot.nhs.uk.
Lorraine Primrose, Email: lorraine.primrose@nhslothian.scot.nhs.uk.
Heather McVicars, Email: heather.mcvicars@nhslothian.scot.nhs.uk.
Pam McKay, Email: pam.mckay@ggc.scot.nhs.uk.
Annabel Borley, Email: annabel.borley@wales.nhs.uk.
Clare Rowntree, Email: clare.rowntree@stoneleigh.me.uk.
Simon Lord, Email: simon.lord@oncology.ox.ac.uk.
Graham Collins, Email: graham.collins@ouh.nhs.uk.
John Radford, Email: john.payne@gjnh.scot.nhs.uk.
Amy Guppy, Email: aguppy@nhs.net.
Michelle C. Williams, Email: michelle.williams@ed.ac.uk.
Alan Japp, Email: alangjapp@hotmail.com.
John R. Payne, Email: john.payne@gjnh.scot.nhs.uk.
David E. Newby, Email: d.e.newby@ed.ac.uk.
Nicholas L. Mills, Email: nick.mills@ed.ac.uk.
Olga Oikonomidou, Email: olga.oikonomidou@ed.ac.uk.
Ninian N. Lang, Email: ninian.lang@glasgow.ac.uk.
REFERENCES
- 1.Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104:971–977. doi: 10.1136/heartjnl-2017-312103 [DOI] [PubMed] [Google Scholar]
- 2.Hamo CE, Bloom MW, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, et al. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9:e002843. doi: 10.1161/CIRCHEARTFAILURE.115.002843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, et al. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9:e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha V, Chandra A, Haykowsky M, Ky B, Moslehi J, et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. JACC CardioOncol. 2019;1:54–65. doi: 10.1016/j.jaccao.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 6.Armenian SH, Lacchetti C, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2017;13:270–275. doi: 10.1200/JOP.2016.018770 [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC [DOI] [PubMed] [Google Scholar]
- 8.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland A, Storås TH, et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hundley WG, D’Agostino R, Zamvar V, Crotts T, Craver K, Hackney MH, Jordan JH, Ky B, Wagner LI, Herrington DM, et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1:10.1056/evidoa2200097. doi: 10.1056/evidoa2200097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel L, Mincu RI, Mrotzek SM, Korste S, Neudorf U, Rassaf T, Totzeck M. Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer-a meta-analysis. ESC Heart Fail. 2020;7:423–433. doi: 10.1002/ehf2.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AS, Anand A, Sandoval Y, Lee KK, Smith SW, Adamson PD, Chapman AR, Langdon T, Sandeman D, Vaswani A, et al. ; High-STEACS Investigators. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet. 2015;386:2481–2488. doi: 10.1016/S0140-6736(15)00391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bularga A, Lee KK, Stewart S, Ferry AV, Chapman AR, Marshall L, Strachan FE, Cruickshank A, Maguire D, Berry C, et al. High-sensitivity troponin and the application of risk stratification thresholds in patients with suspected acute coronary syndrome. Circulation. 2019;140:1557–1568. doi: 10.1161/CIRCULATIONAHA.119.042866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzolos E, Adamson PD, Hall PS, Macpherson IR, Oikonomidou O, MacLean M, Lewis SC, McVicars H, Newby DE, Mills NL, et al. Dynamic changes in high-sensitivity cardiac troponin I in response to anthracycline-based chemotherapy. Clin Oncol (R Coll Radiol). 2020;32:292–297. doi: 10.1016/j.clon.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR, das Dores Cruz F, Gonçalves Brandão SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049 [DOI] [PubMed] [Google Scholar]
- 15.Heck SL, Mecinaj A, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, Røsjø H, Steine K, Geisler J, et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): extended follow-up of a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. 2021;143:2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omland T. Cardio-protective therapy in cardio-oncology: quo vadis? Circulation. 2021;144:667–669. doi: 10.1161/CIRCULATIONAHA.121.055541 [DOI] [PubMed] [Google Scholar]
- 17.Henriksen PA, Hall P, Oikonomidou O, MacPherson IR, Maclean M, Lewis S, McVicars H, Broom A, Scott F, McKay P, et al. Rationale and design of the Cardiac CARE Trial: a randomized trial of troponin-guided neurohormonal blockade for the prevention of anthracycline cardiotoxicity. Circ Heart Fail. 2022;15:e009445. doi: 10.1161/CIRCHEARTFAILURE.121.009445 [DOI] [PubMed] [Google Scholar]
- 18.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ. 2015;350:g7873. doi: 10.1136/bmj.g7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heck SL, Gulati G, Ree AH, Schulz-Menger J, Gravdehaug B, Røsjø H, Steine K, Bratland A, Hoffmann P, Geisler J, et al. Rationale and design of the prevention of Cardiac Dysfunction During an Adjuvant Breast Cancer Therapy (PRADA) trial. Cardiology. 2012;123:240–247. doi: 10.1159/000343622 [DOI] [PubMed] [Google Scholar]
- 21.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144 [DOI] [PubMed] [Google Scholar]
- 22.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.