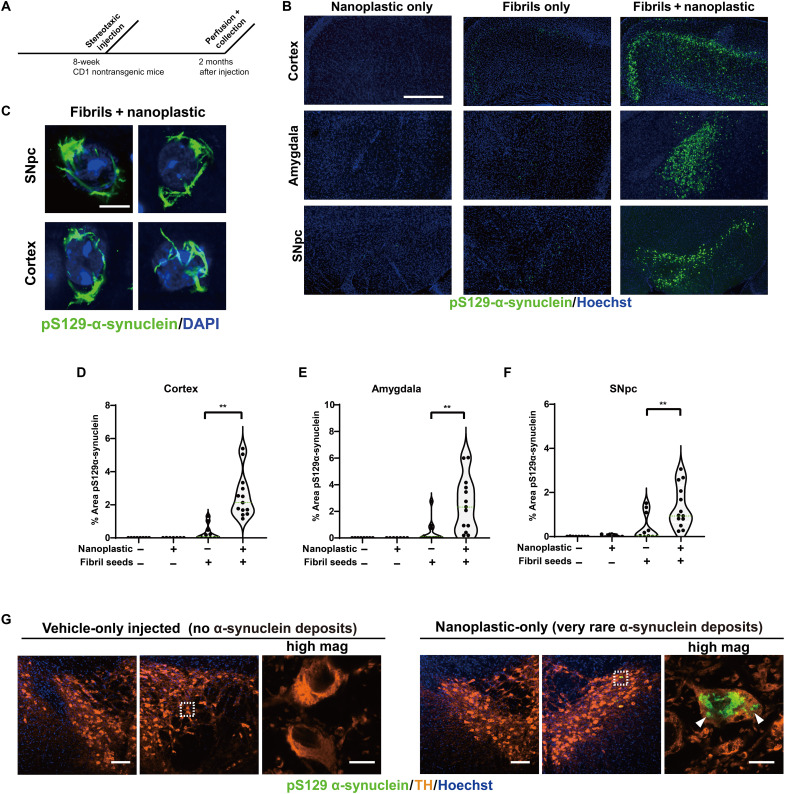
Fig. 9. Anionic nanoplastic contaminants in the brain synergize with α-synuclein fibrils to increase the deposition of pathology in the cortex, amygdala, and substantia nigra in nontransgenic mice.
(A) Timeline for the injection of adult (nontransgenic) male mice with control saline (N = 8 mice), 4.5 μg of unlabeled α-synuclein fibrils (N = 12 mice), or 4.5 μg of unlabeled α-synuclein fibrils combined with 15 μg of unlabeled nanoplastic (N = 12 mice). (B) Representative immunofluorescence staining of α-synuclein pathology (pS129–α-synuclein, green) in the indicated brain region 2 months after injections. Scale bar, 0.5 mm. (C) Representative high-magnification Airyscan images of pS129–α-synuclein staining inside of dopaminergic neurons (SNpc) or pyramidal neurons in the motor cortex (cortex). Scale bar, 10 μm. (D) Violin plots showing the distributed α-synuclein inclusion burden (pS129–α-synuclein area in each image) in ipsilateral cortex, (E) amygdala, and (F) SNpc. Mean percent areas of the indicated brain nuclei occupied by pS129–α-synuclein staining are plotted, where each dot represents the analysis of all sections through the respective brain region of one animal. **P < 0.01, Mann-Whitney U test. (G) Two months after nanoplastic-only injections, 3 of 10 mice demonstrated robust but isolated α-synuclein inclusions (green) in dopaminergic neurons in the SNpc, suggesting that nanoplastic on its own can induce de novo mature inclusions. Bounding boxes are magnified to highlight these inclusions (arrowheads). Scale bars, 100 and 10 μm (for “high mag”).