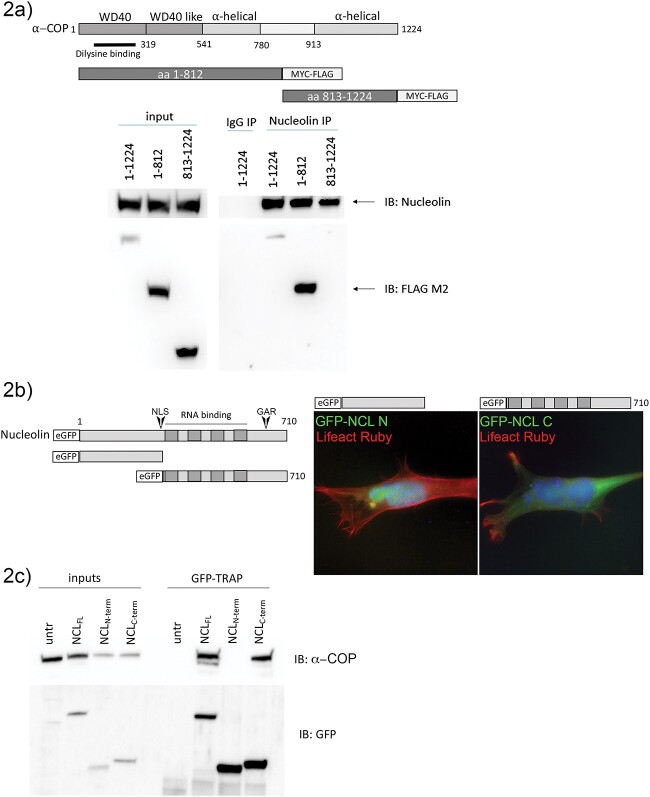
Figure 2.
The N-terminus of α-COP interacts with the C-terminus of Nucleolin. (a) Schematic of α-COP protein structure and Myc-FLAG tagged truncation mutants. A representative Western blot from HEK 293TT cells transfected with full-length, N-terminal or C-terminal α-COP shows that Nucleolin co-immunoprecipitates only full-length or C-terminal α-COP. (b) Schematic of eGFP-tagged Nucleolin and truncation mutants. Representative micrographs from transfected NSC-34 cells show that the N-terminal fragment of Nucleolin which contains the NLS is predominantly nuclear while the C-terminal fragment demonstrates more diffuse cytoplasmic localization. Cells were co-transfected with Lifeact Ruby to visualize cytoarchitecture and nuclei were visualized with DAPI (blue). (c) GFP-TRAP on whole cell lysate from HEK 293TT transfected with full-length, N-terminal or C-terminal eGFP-Nucleolin shows that α-COP clearly co-immunoprecipitates with full-length and C-terminal Nucleolin but not the N-terminal fragment.