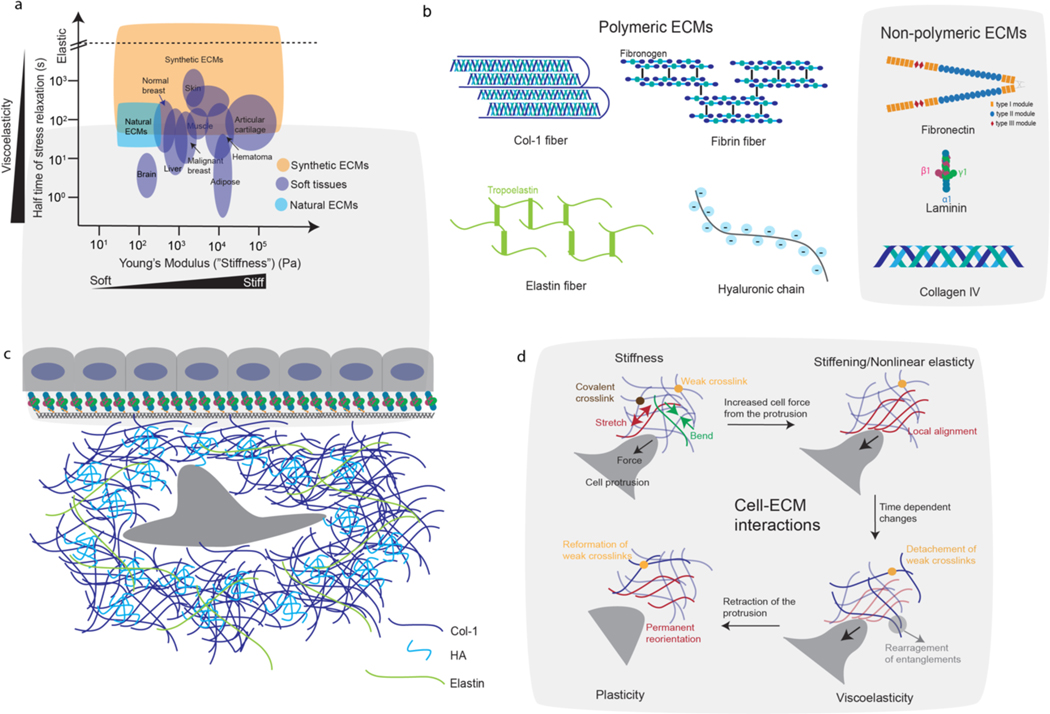
Figure 1. Tissue mechanics, ECM components, and cell-ECM mechanical interactions.
(a) Stiffness and half time of stress relaxation for various soft tissues, and the range of those properties accessible with reconstituted ECMs or synthetic ECMs (i.e. hydrogels). Data were taken from refs. 1,40,52,84,122,135,199,269. Stress relaxation half times, which provide a measure of viscoelasticity, are defined as the time for the stress to relax to half its original value in response to a constant deformation. Synthetic ECMs, including alginate hydrogels and PEG hydrogels, can be modified to mimic the physiological mechanics of soft tissues (check Box 1). (b) Schematics of structural components of major polymeric (left) and non-polymeric (right) ECMs in tissues. (c) Schematic of an epithelial monolayer with basement membrane underneath, and a stromal cell surrounded by fibrous ECMs such as col-1 and elastin in the underlying connective tissue. (d) As cells interact with ECM, these interactions are mediated by mechanical properties of the ECM including stiffness, nonlinear elasticity, viscoelasticity, and plasticity. As the cell push/pull on the ECM, the ECM may resist the cellular force through bending and stretching of the ECM fibers (left top). With increased forces from the cell, the ECM may stiffen (i.e. exhibit greater resistance) due to local alignment in fibers (right top). Over the time of force application, the ECM may undergo creep and stresses may relax due to detachment of weak crosslinks and fiber rearrangements (bottom right). Once the cell detaches from the ECM, the ECM may retain permanent deformations resulting from reformation of weak crosslinks that lock in changes in fibre position and alignment (bottom left).