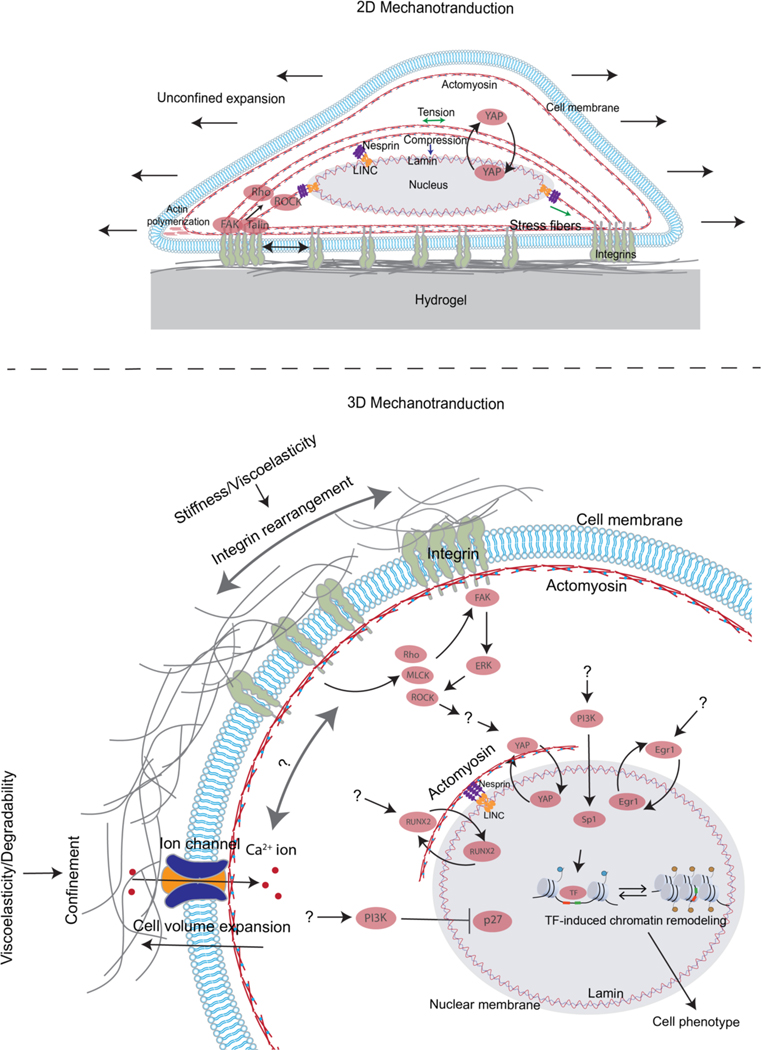
Figure 3. 2D and 3D Mechanotransduction.
A) Cells sense substrate stiffness by exerting contractile forces on 2D substrates with stress fibres through focal adhesions, which activates various proteins such as FAK, talin, Rho and ROCK at the adhesion site. Activation of these proteins leads to adhesion maturation and stress fibre formation and contractility, which in turn transmits forces to the nucleus via the linker of nucleoskeleton and cytoskeleton (LINC) complex, resulting in changes in nuclear envelope tension and nuclear pore opening. This allows the nuclear entry of proteins such as YAP transcriptional regulator leading to downstream impact on cell phenotype. Moreover, in 2D, a cell can spread laterally without encountering any mechanical confinement. B) Cells embedded in the ECM sense stiffness and viscoelastic properties of the matrix through integrin binding, activation, and clustering, while sensing confinement, viscoelasticity and plasticity through cell volume changes and ion channel activation which leads to Ca2+ ion influx. Additionally, ECM stiffness/viscoelasticity and confinement regulate activation of various proteins, such as FAK, ROCK, MLCK, pathways, such as those involving PI3K, ERK, and Rho, and transcriptional regulators such as YAP, p27, Sp1, RUNX2, and EGR1. However, clear mechanistic links between the ECM properties and activation of these proteins, pathways, and transcription regulators remain unclear. Unknown connections in the pathways are indicated by question marks. Both mechanisms of mechanotransduction converge on the nucleus and regulate the activation of transcription factors (TFs), which are facilitated by chromatin remodelling and control cell behaviour.