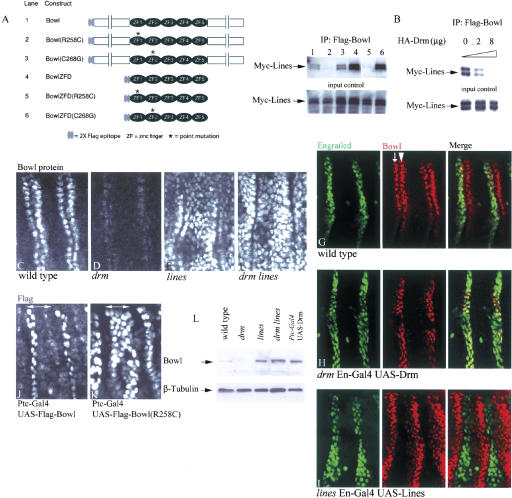
Figure 2.
Lines decreases the steady-state accumulation of Bowl by binding to its N-terminal zinc finger, whereas Drm increases the steady-state accumulation of Bowl by outcompeting the Lines-Bowl interaction. (A) Coimmunoprecipitation of tagged Myc-Lines and Flag-Bowl variants transfected together into S2 cells. Schematic representation of deletion and point mutant variants of Flag-Bowl used in each lane. Lines co-IPed with full-length Bowl protein, its zinc-finger domain—BowlZFD—and a point mutant in the second zinc finger—Bowl(C268G). Lines associated with much reduced affinity with a point mutant in the first finger—Bowl(R258C). A similar point mutation in Drm that disrupts the first finger—Drm(R46C)—reduced interaction with Lines and abolished gain-of-function phenotypes in vivo (Green et al. 2002). (B) Constant amounts of Myc-Lines and Flag-Bowl transfected into S2 cells, together with increasing amounts of Drm: Lines-Bowl interaction was decreased in a dose-dependent manner. (C-F) Bowl immunostains; the boundaries of two PSs are shown. (C) Wild type: Bowl accumulated in cells flanking the segment boundary. (D) drm: Bowl was barely detected. However, Bowl accumulated broadly in lines (E) and drm lines (F). (G-I) Engrailed cells (green), Bowl (red); the right panel is Merge. (G) Wild type: Endogenous Bowl accumulated in cells flanking the segment border, the posteriormost En-expressing cell row (arrow, anterior to the segment border; yellow in H), and in one cell row just posterior to this (arrowhead, posterior to the segment border). (H) drm En-GAL4 UAS-Drm: Bowl accumulated in Drm-expressing cells. Residual Bowl expression was detected in cells anterior to the En domain due to earlier Engrailed expression in these cells. (I) lines En-GAL4 UAS-Lines: Bowl was down-regulated in Lines-expressing cells. (J) Ptc-GAL4 UAS-Flag-Bowl. (K) Ptc-GAL4 UAS-Flag-Bowl(R258C): the Ptc-Gal4 expression domain is marked using a double arrow in J and K. (L) Western blot: 7-11-h AEL embryonic extracts for Bowl from indicated genotypes and β-tubulin as a loading control. Bars: C-K, 10 μm.