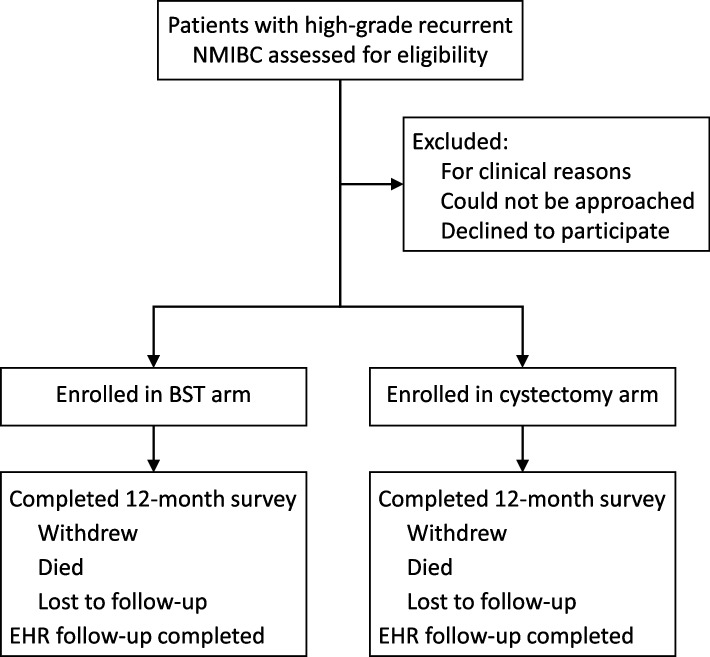
Fig. 3.
CISTO Study overview. Patients with high-grade recurrent non-muscle invasive bladder cancer (NMIBC) seen at urology clinics from the 36 participating CISTO Study sites are assessed for eligibility. Eligible patients are approached for participation and complete an informed consent process. Participants are enrolled in either the bladder-sparing therapy (BST) or cystectomy arm based on their individual treatment decision. All participants are followed for at least 12 months, including by survey and abstraction from the electronic health record (EHR)