FIGURE 4.
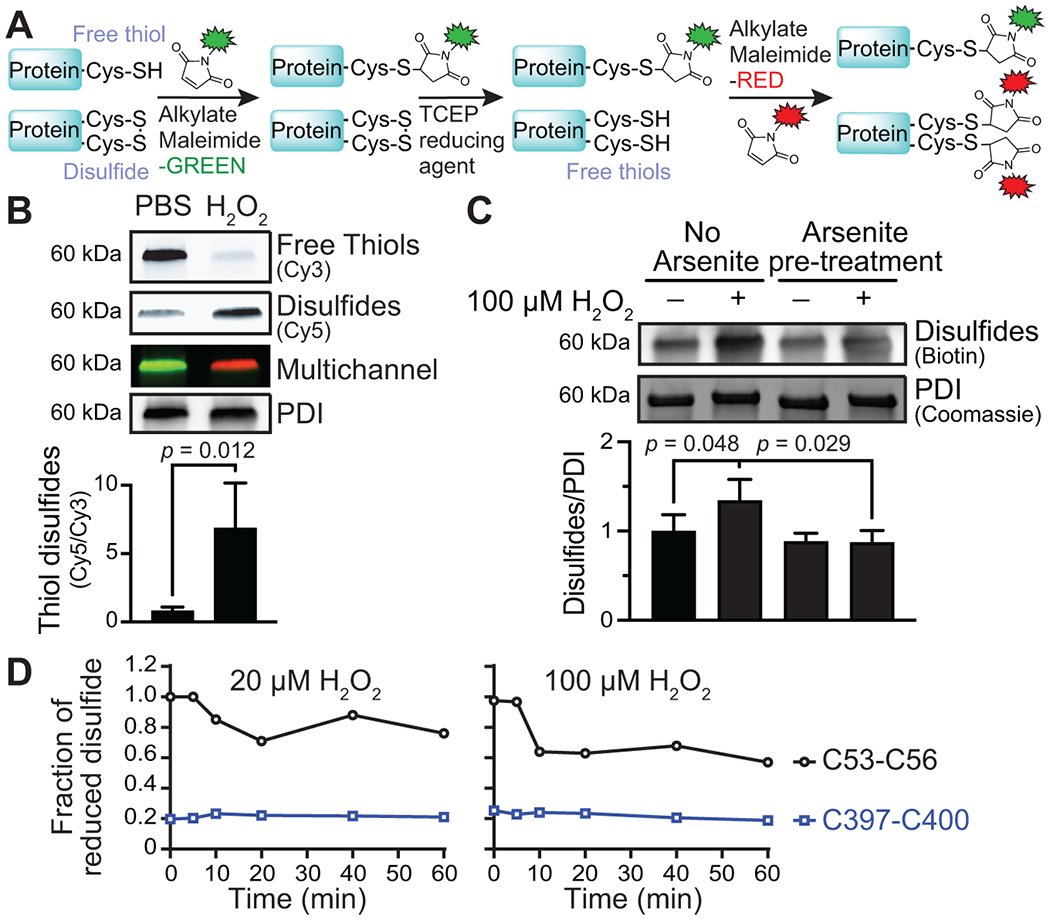
Sulfenylation by H2O2 promotes PDI disulfide formation. (A) Differential alkylation to detect PDI disulfide formation. The free cysteine thiols were labeled and blocked with maleimide-Cy3. Cysteine disulfides and other reversible oxidative modifications, including sulfenylation, were reduced with TCEP, and the newly available free thiols were labeled with maleimide-Cy5. (B) H2O2 promoted disulfide formation on PDI compared to buffer-treated conditions. (C) H2O2-mediated disulfide formation on PDI was prevented by arsenite. (D) Redox state of the PDI a (C53-C56) and a′ (C397-C400) active-site cysteines determined by differential cysteine alkylation and mass spectrometry. p-values were determined by unpaired Student’s t-test in (B) and one-way ANOVA with Tukey’s post hoc analysis in (C). Data represented as mean ± SEM.
