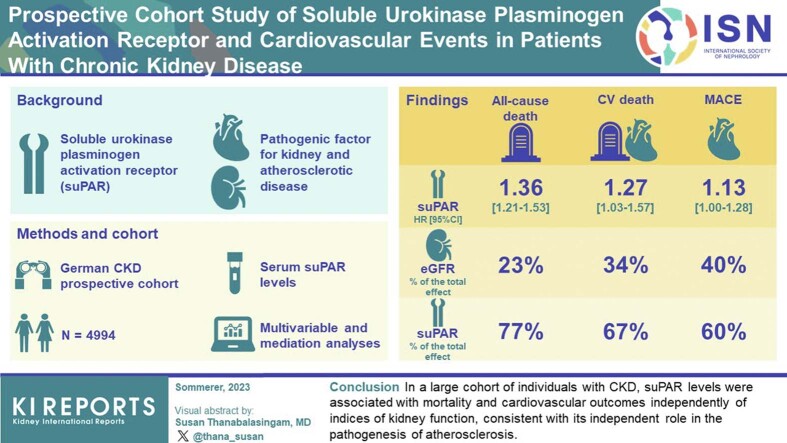
Abstract
Introduction
Soluble urokinase plasminogen activation receptor (suPAR) is an immune-derived pathogenic factor for kidney and atherosclerotic disease. Whether the association between suPAR and cardiovascular (CV) outcomes is dependent on the severity of underlying kidney disease is unclear.
Methods
We measured serum suPAR levels in 4994 participants (mean age 60 years; 60% men; 36% with diabetes mellitus; mean estimated glomerular filtration rate (eGFR) 49 ml/min per 1.73 m2, SD 18) of the German Chronic Kidney Disease (GCKD) cohort and examined its association with all-cause death, CV death, and major CV events (MACE) across the range of eGFR and urine albumin-to-creatinine ratio (UACR).
Results
The median suPAR level was 1771 pg/ml (interquartile range [IQR] 1447–2254 pg/ml). SuPAR levels were positively and independently correlated with age, eGFR, UACR, and parathyroid hormone levels. There were 573 deaths, including 190 CV deaths and 683 MACE events at a follow-up time of 6.5 years. In multivariable analyses, suPAR levels (log2) were associated with all-cause death (hazard ratio [HR] 1.36, 95% confidence interval [CI] 1.21–1.53), CV death (HR 1.27, 95% CI 1.03–1.57), and MACE (HR 1.13, 95% CI 1.00–1.28), and were not found to differ according to diabetes mellitus status, baseline eGFR, UACR, or parathyroid hormone levels. In mediation analysis, suPAR’s direct effect on all-cause death, CV death, and MACE accounted for 77%, 67%, and 60% of the total effect, respectively; whereas the effect mediated through eGFR accounted for 23%, 34%, and 40%, respectively.
Conclusion
In a large cohort of individuals with chronic kidney disease (CKD), suPAR levels were associated with mortality and CV outcomes independently of indices of kidney function, consistent with its independent role in the pathogenesis of atherosclerosis.
Keywords: biomarkers, cardiovascular outcomes, cohort, eGFR, suPAR, uACR
Graphical abstract
People with CKD have a high burden of CV disease (CVD) and are at high risk of events and death from CV causes.1, 2, 3 Despite significant advances in the treatment and prevention of CVD, people with CKD represent a growing population in which the risk of CVD has not been mitigated.4 The risk of CVD in people with CKD goes beyond shared risk factors such as hypertension and diabetes mellitus, with inflammation being explored as a central component of the common pathophysiology of CVD and CKD.5 suPAR may be an important actor of that pathway.
suPAR is an immune-derived signaling glycoprotein involved in the pathogenesis of kidney disease.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 suPAR levels have been shown to be strongly associated with CVD risk factors and outcomes in various populations,9,12,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and have been hypothesized to be a common link between kidney and CVD.24,28, 29, 30, 31 Recently, suPAR has been shown to be causally involved in atherosclerotic disease.32 Whether the association between suPAR and CV outcomes is dependent on kidney function is unclear.
We leveraged the GCKD cohort to better characterize the link between serum suPAR levels, indices of kidney function, and CV outcomes. We hypothesize that suPAR levels are associated with CV outcomes independent of indices of kidney function. GCKD is a large prospective cohort of participants with known CKD who underwent long-term follow-up for CV outcomes and extensive clinical characterization for CVD and indices related to kidney function, including eGFR, UACR, blood phosphate, and intact parathyroid hormone (iPTH) levels.
Methods
Study Design and Cohort
We measured suPAR levels in serum samples collected at enrollment of adult participants of the GCKD cohort; a large prospective observational study of outpatients referred to a nephrologist or at a participating university-affiliated medical center between March 2010 and 2012.33,34 Out of 5217 participants, 4994 (96%) had enough blood samples for suPAR measurements. Patients were enrolled in GCKD if their creatinine-derived eGFR ranged between 30 and 60 ml/min per 1.73 m2 or if they had a urinary albumin excretion of 300 mg/g of creatinine. Glomerular filtration rate values were estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.35 Recipients of organ transplants and patients with cancer or severely symptomatic heart failure (New York Heart Association class IV) were excluded. There were otherwise no restriction or enrollment stratification based on the underlying cause of kidney disease. The study was approved by local ethics committees and registered in the national registry for clinical studies (DRKS 00003971) and conducted in accordance with the principles of the Declaration of Helsinki. Informed written consent was obtained from all participants.
Follow-up and Outcomes
Patients underwent annual interviews by trained personnel in alternating phone visits and face-to-face interactions. For the purpose of this analysis, data extraction from the main GCKD database was performed in February 2022. The median follow-up time was 6.5 years; 499 (2.3 %) participants had been lost to follow-up, and 311 (5.9%) of participants opted out of the study. Data on all-cause mortality, and CV events were collected and adjudicated based on medical reports obtained from hospitalizations, death certificates, and autopsies. The outcomes examined were all-cause death; CV death (fatal myocardial infarction, sudden cardiac death, and death due to nonhemorrhagic stroke); and MACE, defined as the composite of CV death, nonfatal myocardial infarction, hospitalization due to angina or heart failure, cardiac surgery, stroke, and peripheral arterial disease (PAD) events (amputation due to PAD, including diabetes-related secondary disease, surgical or percutaneous revascularization due to PAD, or death due to PAD).
suPAR Measurements
We measured plasma levels of suPAR using the human suPAR Quantikine ELISA Kit (R&D systems, Minneapolis, MN) according to the manufacturer's protocol (Catalog Number DUP00). The minimal detectable suPAR concentration was 33 pg/ml. The intraassay coefficients of variation for low (mean 836 pg/ml), medium (1593 pg/ml), and high (2412 pg/ml) suPAR levels were 2.1%, 4.1%, and 7.5%, respectively. SuPAR measurements have been shown to be stable in long-term storage (over 5 years) and are minimally affected by repeated freezing and thawing cycles.36 Technicians measuring suPAR were blinded to clinical data.
Statistical Analysis
Clinical Characteristics, Kidney Function, and suPAR Levels
We described the cohort using mean values and SDs for normally distributed variables, and median values and IQRs (25th – 75th percentile) for nonnormally distributed variables. Categorical variables were presented as frequency distributions with percentages. Descriptive analyses were performed for both the overall cohort and for strata defined by MACE occurrence and suPAR quintiles. We used boxplots depicting suPAR levels across eGFR categories (<30, 30–45, 46–60, >60 ml/min per 1.73 m2) and severity of albuminuria (<30 mg/l, 30–300 mg/l, or >300 mg/l). We reported the Spearman rank correlation between suPAR, eGFR, UACR, and iPTH. To identify determinants of suPAR levels, we used linear regression modeling of log2(suPAR) (dependent variable), including the following covariates in the model: age, gender, body-mass index, eGFR, UACR, smoking, hypertension, diabetes mellitus, coronary artery disease, PAD, history of stroke, statin therapy, low density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels. Missing data was <1% for the aforementioned variables.
suPAR and Outcomes
Time-to-event outcomes were defined as follows: If patients failed to complete the 6-year follow-up period, censoring was performed at the time of the last follow-up; for example, when participants were lost to follow-up or refused to further participate in the study. We plotted cumulative incidence functions for each of the analyzed outcomes by applying the Aalen-Johansen estimator.
After checking that the proportional hazard assumption was not violated (supremum test, including a correction for multiple comparisons by the Bonferroni-Holm method), we used Cox proportional hazards regression to characterize the association between suPAR levels at enrollment and the 3 outcomes, namely all cause death, CV death, and MACE as previously defined. Due to its skewed distribution, suPAR levels were examined both as a continuous variable (log2 transformed) and as a categorical variable (quintiles). The following nested models were examined: Model 0 included suPAR alone; model 1 included suPAR in addition to demographics and CV risk factors: age, gender, body-mass index, current smoking, hypertension, diabetes mellitus, low density lipoprotein, and high-density lipoprotein. In model 2, we added coronary artery disease, stroke, and PAD. Model 3 included in addition indices of kidney function, namely eGFR and UACR. Lastly, model 4 included phosphate and iPTH levels. HRs obtained from Cox regression were presented with 95% CI. We created a forest plot depicting the HR of suPAR in each model.
Sensitivity and Mediation Analyses
To assess whether the association between suPAR and outcomes differed according to subgroups of patients with CKD, we tested the interaction between the log2-transformed suPAR level and the variables of interest, and computed HR for each outcome in the following subgroups: patients with and without diabetes mellitus, patients stratified according to eGFR (<30, 30–45, 46–60, >60 ml/min per 1.73 m2) and albuminuria (<30 mg/l, 30–300 mg/l, >300 mg/l). In order to ascertain whether the influence of suPAR on all-cause mortality, CV mortality, and MACE was mediated by eGFR and UACR, a causal mediation analysis was conducted employing the variables from model 4. To accomplish this, we utilized the 'mediate()' function available in the ‘mediation’ package in R. In this process, we deployed a model-based strategy, which encompassed 1000 simulations and leveraged a quasi-Bayesian approach for the estimation of standard errors. In our report, we detailed estimates of the average causal mediation effect, a numerical measure denoting the extent of the mediator's influence on the overall effect of the exposure-outcome association. In addition, we provided estimates of the average direct effect, the average total effect, and the proportion mediated.
Analyses were performed using SAS 9.4 2002–2012 (SAS Institute Inc., Cary, NC) and R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Cohort Characteristics
A total of 4994 participants of the GCKD cohort were included in this analysis (Table 1). The cohort consisted of 60.1% men. Over a third of participants (35.9%) had diabetes mellitus, and 19.8% had known coronary artery disease. The mean eGFR of the cohort was 49.4 (SD 18.3) ml/min per 1.73 m2, and 23.2% of participants had >300 mg/l of albuminuria. The median serum phosphate level was 1.10 mmol/l (IQR 0.97–1.24), and the median intact parathormone level was 37.1 pg/ml (IQR 24.0–58.1). Detailed clinical characteristics are listed in Table 1.
Table 1.
Cohort characteristics stratified by MACE
| Characteristics | All (N = 4994) | MACE-free Survivors (N = 4311) | MACE (N = 683) |
|---|---|---|---|
| Age (yrs), mean (SD) | 60 (12) | 59 (12) | 65 (8) |
| Male, n (%) | 3001 (60.1) | 2484 (49.7) | 517 (75.7) |
| Body mass index (kg/m2), mean (SD) | 30 (6) | 30 (6) | 31 (6) |
| History of smoking, n (%) | 2951 (59.1) | 2486 (57.7) | 465 (68.1) |
| eGFR (ml/min per 1.73 m2), mean (SD) | 49 (18) | 50 (19) | 44 (15) |
| UACR, urine (mg/g), (median; 25th–75th percentile) | 50.9 (10–387) | 49 (9–375) | 530 (47–510) |
| Albuminuria n (%) | |||
| <30 mg/l | 2363 (48.1) | 2066 (47.9) | 297 (43.5) |
| 30–300 mg/l | 1412 (28.7) | 1210 (28.0) | 202 (29.6) |
| >300 mg/l | 1141 (23.2) | 966 (22.4) | 175 (25.6) |
| Hypertension, n (%) | 4807 (96.3) | 4132 (95.8) | 675 (98.8) |
| Diabetes mellitus, n (%) | 1791 (35.9) | 1387 (32.2) | 404 (59.2) |
| Peripheral arterial disease, n (%) | 471 (9.4) | 319 (7.3) | 152 (22.3) |
| Coronary artery disease, n (%) | 988 (19.8) | 705 (16.3) | 283 (41.4) |
| Stroke, n (%) | 481 (9.6) | 348 (8.1) | 133 (19.5) |
| Heart failure, n (%) | 884 (17.7) | 699 (16.2) | 185 (27.1) |
| Statin treatment, n (%) | 2359 (47.2) | 1945 (38.9) | 414 (60.6) |
| LDL (mg/dl), median;(25th–75th percentile) | 114 (89–143) | 115 (91–144) | 103 (80–135) |
| HDL (mmol/l), median;(25th–75th percentile) | 48 (39–61) | 49 (40–62) | 44 (35–55) |
| Phosphate (mmol/l), median (25th–75th percentile) | 1.11 (0.97–1.24) | 1.10 (0.97–1.23) | 1.11 (0.97–1.24) |
| iPTH (pg/ml), median (25th–75th percentile) | 37.1 (24.0–58.1) | 36.5 (23.8–56.5) | 44.25 (29.5–71.8) |
| SuPAR (pg/ml), median (25th–75th percentile) | 1771 (1447–2254) | 1745 (1426–2219) | 1959 (1608–2479) |
eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL, low density lipoprotein cholesterol; MACE, major adverse cardiovascular events; suPAR, soluble urokinase plasminogen activator receptor; UACR, urine albumin-to-creatinine ratio.
suPAR Level and Their Determinants
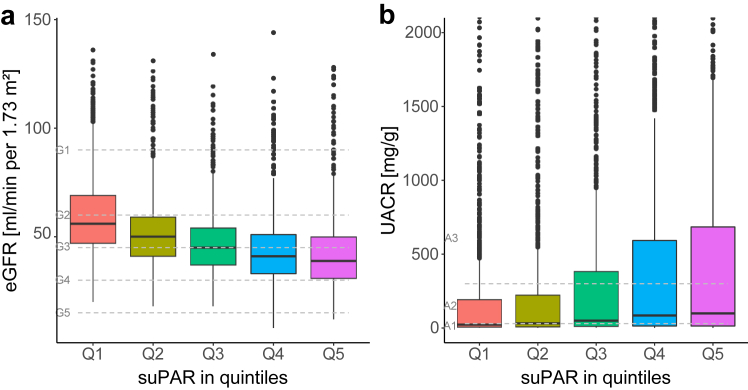
The median suPAR level of the cohort was 1771 pg/ml (IQR, 1447–2254). SuPAR levels tended to be higher in women; active smokers; and patients with hypertension, diabetes mellitus, and atherosclerotic disease (Table 2). We found a negative correlation between suPAR levels and eGFR (rs = -0.38), and a weak positive correlation with UACR (rs = 0.17), phosphate (rs = 0.10) and iPTH (rs =0.24). Participants in the highest suPAR quintile (>2417 pg/ml) had a lower mean eGFR (43 [SD 17] ml/min per 1.73 m2) and higher median UACR (99 mg/g IQR[13–685]) compared to those in the lowest suPAR quintile (≤1378 pg/ml), with an eGFR of 61 (SD 20) ml/min per 1.73 m2 and UACR of 23 mg/g (IQR 6–192) (Table 2). The increase in suPAR levels across eGFR and albuminuria categories was stepwise (Figure 1). In multivariable analysis adjusting for clinical characteristics and indices of kidney function, we found suPAR levels to be independently associated with age, diabetes mellitus, eGFR, UACR and iPTH (Table 3).
Table 2.
Cohort characteristics stratified by soluble urokinase plasminogen activator receptor quintiles
| Characteristics | suPAR Quintile 1 ≤1378 |
suPAR Quintile 2 >1378 – ≤1636 |
suPAR Quintile 3 >1636 – ≤1929 |
suPAR Quintile 4 >1929 – ≤2417 |
suPAR Quintile 5 >2417 |
|---|---|---|---|---|---|
| (N = 999) | (N = 1000) | (N = 998) | (N = 999) | (N = 998) | |
| Age (yrs), mean (SD) | 58 (12) | 60 (12) | 61 (12) | 62 (11) | 60 (13) |
| Gender, male n (%) | 669 (67.0) | 602 (60.2) | 566 (56.7) | 585 (58.6) | 579 (58.0) |
| Body mass index (kg/m2), mean (SD) | 29 (5) | 30 (6) | 30 (6) | 30 (6) | 30 (7) |
| History of smoking, n (%) | 556 (55.6) | 566 (56.6) | 561 (56.2) | 654 (65.5) | 614 (61.5) |
| eGFR (ml/min per 1.73 m2), mean (SD) | 61 (20) | 52 (17) | 47 (15) | 44 (16) | 43 (17) |
| UACR (mg/g), median (25th–75th percentile) | 24 (6–192) | 33 (8–223) | 51 (11–383) | 85 (14–593) | 99 (13–685) |
| Albuminuria, n (%) | |||||
| <30 mg/l | 552 (56.4) | 536 (54.6) | 459 (47.0) | 417 (42.3) | 399 (40.5) |
| 30-300 mg/l | 253 (25.9) | 270 (27.3) | 298 (30.5) | 292 (29.6) | 299 (30.3) |
| >300 mg/l | 173 (17.7) | 182 (18.4) | 220 (22.5) | 278 (28.2) | 288 (29.2) |
| Hypertension, n (%) | 935 (93.6) | 963 (96.5) | 963 (96.5) | 980 (98.2) | 966 (96.8) |
| Diabetes mellitus, n (%) | 252 (25.2) | 300 (30.0) | 375 (37.6) | 436 (43.6) | 428 (42.9) |
| Peripheral arterial disease, n (%) | 52 (5.21) | 74 (7.41) | 97 (9.72) | 120 (12.0) | 128 (12.8) |
| Coronary artery disease, n (%) | 132 (13.2) | 170 (17.0) | 222 (22.2) | 248 (24.6) | 216 (21.6) |
| Stroke, n (%) | 67 (6.71) | 95 (9.51) | 93 (9.32) | 118 (11.8) | 108 (10.8) |
| Heart failure, n (%) | 123 (12.3) | 171 (17.1) | 179 (17.9) | 211 (21.1) | 200 (20.0) |
| Statin treatment, n (%) | 411 (41.1) | 453 (45.3) | 494 (49.5) | 530 (53.1) | 471 (47.2) |
| LDL (mg/dl), median (25th–75th percentile) | 118 (96–146) | 117 (92–143) | 114 (87–143) | 110 (86–142) | 109 (84–139) |
| HDL (mmol/l), median (25th–75th percentile) | 50 (41–61) | 49 (40–62) | 48 (40–62) | 47 (38–60) | 47 (37–61) |
| Phosphate (mmol/l), median (25th–75th percentile) | 1.08 (0.96–1.20) | 1.10 (0.96–1.22) | 1.09 (0.96–1.22) | 1.11 (0.99–1.25) | 1.14 (0.99–1.28) |
| iPTH (pg/ml), median (25th–5th percentile) | 29.2 (20.1–41.5) | 33.9 (22.3–50.9) | 38.9 (24.9–58.6) | 42.3 (27.9–67.7) | 46.5 (27.0–72.0) |
eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL, low density lipoprotein cholesterol; suPAR, soluble urokinase plasminogen activator receptor; UACR, urine albumin-to-creatinine ratio.
Figure 1.
Estimated glomerular filtration rates (eGFR) and urine albumin-to-creatinine ratio (UACR) stratified by suPAR quintiles. Box plots depicting the distribution of eGFR and UACR stratified by suPAR quintiles. Panel A shows the distribution of eGFR across suPAR quintiles. Panel B shows the distribution of UACR across suPAR quintiles. The box represents the interquartile range (IQR), with the line inside the box indicating the median value. Whiskers extend to 1.5 times the IQR. suPAR, soluble urokinase plasminogen activation receptor
Table 3.
Determinants of suPAR levels in GCKD
| Variables | SuPAR, log-transformed |
||
|---|---|---|---|
| Estimate | 95% CI | P value | |
| Age, per yr | −0.0052 | (−0.0070 to −0.0035) | <0.001 |
| Male gender | 0.0999 | (−0.1410 to −0.0587) | <0.001 |
| Body mass index, per 1 kg/m2 | 0.0004 | (−0.0029 to 0.0037) | 0.81 |
| Current smoking | 0.0081 | (−0.0290 to 0.0452) | 0.67 |
| eGFR, per 1 ml/min per 1.73 m2 | −0.0082 | (−0.0093 to −0.0070) | <0.001 |
| UACR, per 1 mg/g | 0.0001 | (0.000 to 0.0001) | <0.001 |
| Hypertension | 0.0571 | (−0.0378 to 0.1519) | 0.24 |
| Diabetes mellitus | 0.0838 | (0.0427 to 0.1250) | <0.001 |
| Peripheral arterial disease | 0.0855 | (0.0239 to 0.1472) | 0.007 |
| Coronary artery disease | 0.0218 | (−0.0276 to 0.0712) | 0.38 |
| Stroke | 0.0449 | (−0.0158 to 0.1057) | 0.15 |
| Heart failure | 0.0303 | (−0.0197 to 0.0803) | 0.06 |
| Low density lipoprotein, per 1 mg/dl | −0.0006 | (−0.0011 to −0.0002) | 0.005 |
| High density lipoprotein, per 1 mg/dl | 0.0006 | (−0.0005 to 0.0017) | 0.29 |
| Phosphate, per 1 mmol/l | 0.0796 | (−0.0116 to 0.1708) | 0.09 |
| iPTH, per 1 pg/ml | 0.0015 | (0.0010 to 0.0020) | <0.001 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; GCKD, German Chronic Kidney Disease cohort; HDL, high density lipoprotein cholesterol; iPTH, intact parathyroid hormone; LDL, low density lipoprotein cholesterol; suPAR, soluble urokinase plasminogen activator receptor; UACR, urine albumin-to-creatinine ratio.
Outcomes in GCKD
Overall, there were 573 deaths, including 190 CV deaths and 683 MACE events after a median follow-up time of 6.5 years. Participants who experienced a MACE at follow-up were older, more likely to be male, with a history of smoking, and a higher prevalence of comorbidities (Table 1). The median suPAR level was higher in participants who experienced MACE (1959 pg/ml [IQR 1608–2479]), compared to 1745 pg/ml (1426–2219) in MACE-free survivors. In multivariable analysis, independent predictors of MACE included age, gender, smoking, diabetes mellitus, coronary artery disease, PAD, a history of stroke, high-density lipoprotein, LDL, UACR, eGFR, iPTH, and suPAR levels (Table 4). Predictors of all-cause death and CV death and their estimates are also provided in Table 4.
Table 4.
Independent predictors of all-cause death, cardiovascular death, and MACE
| Variables | Hazard ratio, (95% CI) |
||
|---|---|---|---|
| All cause death (n = 573) | Cardiovascular death (n = 190) | MACE (n = 683) | |
| SuPAR, per log-base 2 | 1.36 (1.21–1.53) | 1.27 (1.03–1.57) | 1.13 (1.00–1.28) |
| Age, per yr | 1.06 (1.05–1.07) | 1.06 (1.03–1.08) | 1.04 (1.03–1.05) |
| Female vs. male | 0.56 (0.44–0.70) | 0.48 (0.32–0.73) | 0.58 (0.48–0.72) |
| Body mass index, per 1 kg/m2 | 1.00 (0.99–1.02) | 1.02 (0.99–1.04) | 1.00 (0.99–1.02) |
| Current smoking | 2.05 (1.57–2.69) | 1.19 (0.73–1.95) | 1.64 (1.29–2.08) |
| eGFR, per 1 ml/min per 1.73 m2 | 0.99 (0.98–1.00) | 0.98 (0.97–0.99) | 0.99 (0.99–1.00) |
| UACR, per 1 mg/g | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Hypertension | 1.15 (0.47–2.82) | 0.67 (0.16–2.78) | 1.31 (0.62–2.79) |
| Diabetes mellitus | 1.89 (1.56–2.29) | 2.37 (1.68–3.34) | 1.90 (1.60–2.26) |
| Peripheral arterial disease | 1.35 (1.08–1.70) | 1.38 (0.94–2.03) | 2.15 (1.77–2.60) |
| Coronary artery disease | 1.32 (1.09–1.60) | 2.09 (1.51–2.89) | 1.84 (1.55–2.19) |
| Stroke | 1.46 (1.17–1.82) | 1.36 (0.92–2.00) | 1.64 (1.35–2.01) |
| Heart failure | 1.72 (1.42–2.10) | 1.48 (1.07–2.07) | 1.12 (0.93–1.35) |
| Low density lipoprotein cholesterol, per 1 mg/dl | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.00) |
| High density lipoprotein cholesterol, per 1 mg/dl | 1.00 (0.99–1.01) | 0.99 (0.97–1.00) | 0.99 (0.99–1.00) |
| Phosphate, per 1 mmol/l | 0.98 (0.63–1.52) | 1.26 (0.59–2.69) | 1.35 (0.90–2.02) |
| iPTH, per 1 pg/ml | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.00) |
CI, confidence interval; eGFR, estimated glomerular filtration rate; iPTH, intact parathyroid hormone; LDL, low density lipoprotein cholesterol; suPAR, soluble urokinase plasminogen activator receptor; UACR, urine albumin-to-creatinine ratio.
suPAR and Outcomes
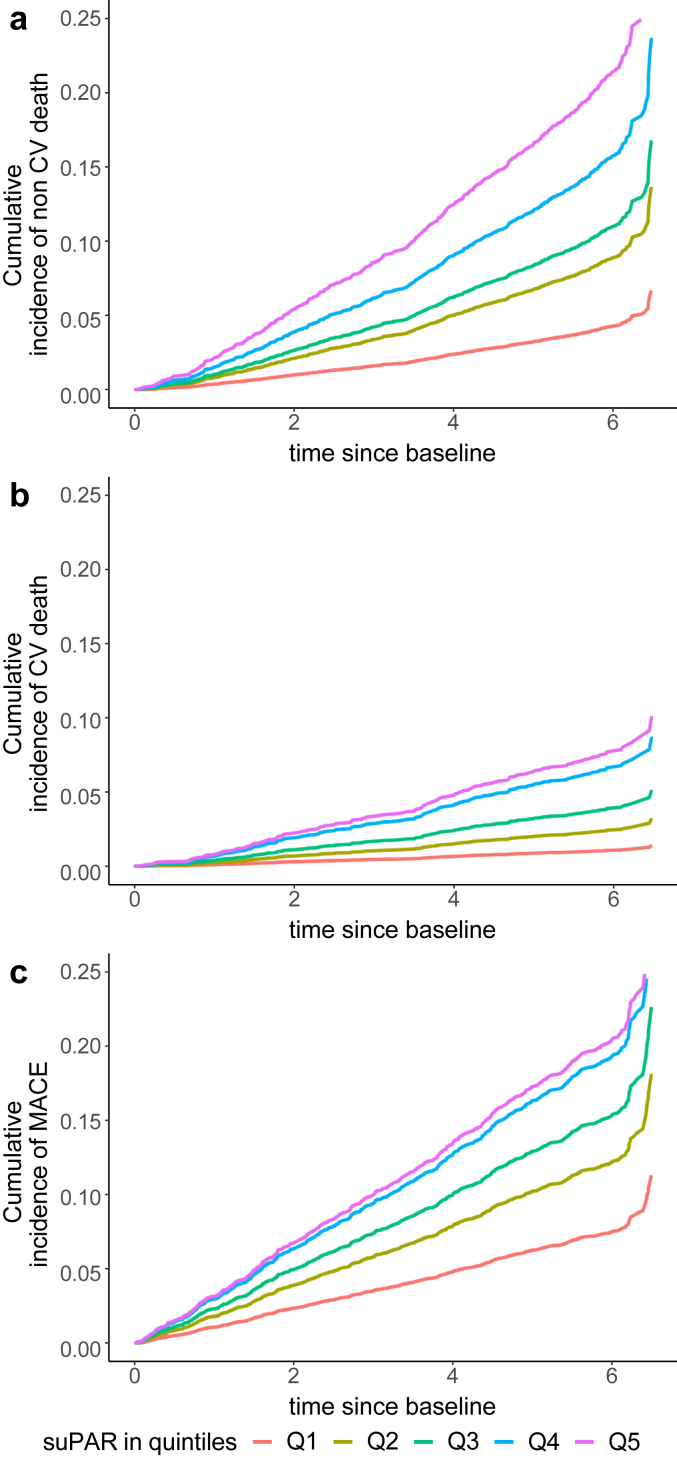
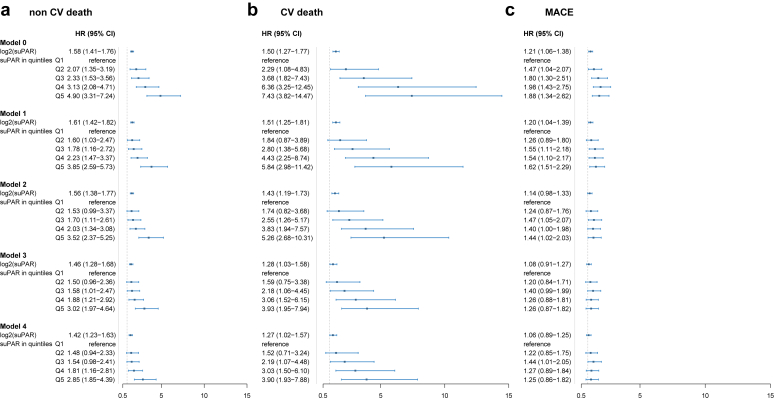
In univariable analysis (model 0), we noted a stepwise increase in the risk of MACE, all-cause death and CV death associated with suPAR quintiles (Figures 2 and 3). The highest suPAR quintile (>2417 pg/ml) was associated with a 2.9-fold (95% CI 2.2–3.8) increase in the risk of MACE, 5.5-fold (95% CI 3.9–7.7) increase in the risk of all-cause death, and 7.4-fold (95% CI 3.8–14.4) increase in the risk of CV death compared to the lowest quintile (≤1378 pg/ml) (Figure 3). The association between suPAR levels and outcomes exhibited minimal attenuation after adjusting for demographics and CV risk factors or phosphate and iPTH levels (Figure 3). The strongest attenuation of the association occurred after adjusting for eGFR and UACR. Overall, suPAR levels remained associated with all-cause death, CV death and MACE after full adjustment for demographics, CV risk factors, prior CVD, and indices of kidney function (Figure 3).
Figure 2.
Cumulative incidence of outcomes stratified by suPAR quintiles. (a) shows the cumulative incidence of all-cause death according to suPAR quintiles. (b) shows the cumulative incidence of cardiovascular death according to suPAR quintiles. (c) shows the cumulative incidence of MACE according to suPAR quintiles. MACE, major adverse cardiovascular events; suPAR, soluble urokinase plasminogen activation receptor.
Figure 3.
Forest plots depicting the association between suPAR levels and outcomes. 3-panel forest plot depicting the hazard ratios and 95% confidence interals of the association between each quintile of suPAR (the reference being quintile 1), and (a) all-cause death, (b) cardiovascular death, and (c) major adverse cardiovascular events. Model 0 included suPAR alone; model 1 included suPAR in addition to demographics and CV risk factors: age, gender, body mass index, current smoking, hypertension, diabetes mellitus, LDL, and HDL. In model 2, we added coronary artery disease, stroke and PAD. Model 3 included indices of kidney function eGFR and UACR. Lastly, model 4 included phosphate and iPTH levels. CV, cardiovascular; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; iPTH, intact parathyroid hormone; LDL, low-density lipoprotein; PAD, peripheral artery disease; suPAR, soluble urokinase plasminogen activation receptor; UACR, urine albumin-to-creatinine ratio.
Sensitivity and Mediation Analysis
We found no interaction between suPAR and diabetes status, baseline eGFR, UACR, or iPTH in the association with all-cause death, CV death and MACE (P > 0.1 for all interaction terms).
In unadjusted mediation analysis, the total effect of suPAR for all-cause death was 0.063 (95% CI 0.050–0.080), 0.019 for CV death (95% CI 0.011–0.030) and 0.032 for MACE (95% CI 0.019–0.050). SuPAR’s direct effect on all-cause death, CV death and MACE accounted for 77%, 67%, and 60% of the total effect ,respectively; whereas the effect mediated through eGFR accounted for 23%, 34%, and 40%, respectively. After accounting for all the aforementioned confounders (model 4), the proportion of the effect mediated by eGFR and UACR remained small, whereas suPAR’s direct effect on all-cause death, CV death, and MACE accounted for 97%, 89%, and 89% of the total effect, respectively and relative to eGFR (Table 5). Results were similar for UACR (Table 5).
Table 5.
Causal mediation analysis of suPAR on all-cause death, cardiovascular death, and MACE
| Variables | Mediator | Estimate, (95% CI) |
|||
|---|---|---|---|---|---|
| Total effect | ACME | ADE | Proportion Mediated | ||
| All-cause death | eGFR | 0.0431 (0.0283; 0.0579) | 0.0012 (−0.0011; 0.0035) | 0.0419 (0.0275; 0.0566) | 0.0274 (−0.0290; 0.0927) |
| UACR | 0.0412 (0.0270; 0.0548) | 0.0017 (0.0003; 0.0031) | 0.0395 (0.0250; 0.0538) | 0.0402 (0.0079; 0.0845) | |
| CV death | eGFR | 0.0123 (0.0049; 0.0191) | 0.0014 (0.00001; 0.0029) | 0.0109 (0.0032; 0.0178) | 0.1115 (0.0004; 0.3531) |
| UACR | 0.0121 (0.0051; 0.0189) | 0.0007 (−0.0002; 0.0017) | 0.0114 (0.0045; 0.0184) | 0.0576 (−0.0181; 0.2014) | |
| MACE | eGFR | 0.0169 (0.0037; 0.0303) | 0.0019 (−0.0005; 0.0046) | 0.0150 (0.0014; 0.0287) | 0.1090 (−0.0317; 0.5965) |
| UACR | 0.0165 (0.0036; 0.0301) | 0.0015 (0.0001; 0.0030) | 0.0165 (0.0036; 0.0301) | 0.0904 (0.0039; 0.4151) | |
ACME, average causal mediation effect; ADE, average direct effect; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; MACE, major adverse cardiovascular events; suPAR, soluble urokinase plasminogen activator receptor; UACR, urine albumin-to-creatinine ratio.
The model included age, gender, body-mass index, current smoking, hypertension, diabetes mellitus, low-density lipoprotein levels, high-density lipoprotein levels, coronary artery disease, stroke, peripheral arterial disease, phosphate and intact parathormone.
Discussion
In this large prospective CKD cohort study, we comprehensively characterize the association between suPAR, mortality and CV outcomes in patients with CKD. Our study includes nearly 5000 participants with long-term follow-up and accounts for several indices of kidney function, including eGFR, UACR, and iPTH. SuPAR levels were significantly associated with all-cause death, CV death, and MACE, independent of clinical characteristics and the aforementioned indices of kidney function. Importantly, the association did not differ according to baseline eGFR or UACR. Furthermore, mediation analysis revealed that less than one-third of the effect of suPAR on all-cause death and CV death was mediated through eGFR. The findings confirms that suPAR’s link to adverse CV outcomes is only partially related to its involvement in the pathophysiology of kidney disease and supports recent findings of suPAR’s independent role in atherosclerotic disease.32
The association between kidney disease and atherosclerotic disease is well-established, with both conditions sharing several risk factors, including hypertension, dyslipidemia, and diabetes.37,38 Patients with CKD have an increased risk of developing CVD, and CVD is a leading cause of morbidity and mortality in this population.39,40 Several studies have shown that CKD is an independent predictor of adverse CV outcomes, such as myocardial infarction and stroke, even after adjusting for common risk factors.39,40 Mechanistically, CKD is associated with several abnormalities in lipid metabolism, endothelial function, and vascular calcification that contribute to the development and progression of atherosclerosis. Well-established therapies for CVD such as statins and antiplatelet agents have debatable effectiveness in reducing the CV risk of patients with CKD, which remains high despite optimization of risk factors in this patient population. Therefore, the identification of novel biomarkers and therapeutic targets that can reduce the burden of atherosclerotic disease in patients with CKD represents a critical unmet need in nephrology research.
Inflammation is a common pathophysiologic link between renal and CVDs and is being explored as a therapeutic target for both disease states.41 SuPAR has been identified as an inflammatory mediator of renal-CV interactions due to its involvement in the pathogenesis of both disease states.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16,32 The urokinase receptor system is versatile and involved in the regulation of various aspects of innate and adaptive immune activity including cell proliferation, differentiation, adhesion and migration, with levels of suPAR thought to reflect the aggregate of its activity.42 SuPAR levels have been consistently associated with atherosclerotic disease and CV outcomes across patient populations,17,21,24,43, 44, 45 and more strongly than several other inflammatory and CV biomarkers, including C-reactive protein, interleukin-6, and NT-proBNP.24,46 Although suPAR was initially perceived to be a biomarker of inflammation and decreased renal function, recent genetic and experimental evidence strongly supports a pathogenic role for suPAR in atherosclerotic disease through the modulation of monocyte and macrophage physiology, that is distinct from its involvement in kidney disease.32 Our analysis from the GCKD cohort provides additional supportive evidence through showing that suPAR levels remain associated with CV events across the spectrum of eGFR and UACR. Mediation analysis showed that most of the effect is direct, rather than mediated through eGFR. This is consistent with a previous study in patients on dialysis, in whom suPAR was also predictive of CV outcomes despite the absence of renal function.31 Only a small proportion of suPAR is cleared by the kidney,47 implying that the increase in suPAR in patients with CKD is likely due to an active pathogenic process rather than decreased clearance. Together, these data place suPAR as a pathogenic link between CKD and atherosclerotic disease, with rising suPAR levels in patients with CKD leading to both a decline in kidney function as well as CV events.
Strengths and Limitations
There are several strengths to our study. The GCKD cohort study enrolled participants with CKD and a range of kidney function, who are under nephrological care, across 9 large regions in Germany. The large sample size and long follow-up allows for adjustment for important confounders and performing subgroup analyses. Biological sample collection was prespecified within the study and therefore occurred systematically for all participants. All parameters were measured in a central laboratory. The main limitation of the cohort is in the lack of non-White representation in the cohort, limiting generalizability across race. SuPAR was measured using the Quantikine assay, which may have led to underestimation of the effect size of the association between suPAR and outcomes as recently shown.48,49 We are unable to differentiate between the different subphenotypes of CKD in GCKD, which may have some implications because the strength of the association between suPAR and kidney disease may differ by these phenotypes.
In conclusion, suPAR levels are associated with CV outcomes independent of indices of kidney function and across the range of eGFR and UACR levels. Mediation analysis confirms a predominantly direct effect of suPAR on CV death. Our findings are in line with recently published work on suPAR’s causal role in atherosclerosis independent of its known involvement in CKD. With the advent of anti-suPAR therapies, our study contributes to a better understanding of the complex interplay between suPAR and CV outcomes in patients with CKD and highlights the potential clinical implications of targeting suPAR as a therapeutic approach to reduce CV risk in this population.37
Disclosure
CS, CN, SMK, JN, KUE, MS, and MZ have no conflicts of interest to report concerning the present publication. SSH and JR are scientific advisory board members of Walden Biosciences, a company devising therapeutics targeting suPAR in kidney disease.
Acknowledgments
The authors are very grateful for the willingness and time of all study participants of the GCKD study. The enormous effort of the study personnel at the regional centers is highly appreciated. The authors also thank the large number of nephrologists for their support of the GCKD study. The GCKD study is supported by the German Ministry of Education and Research (Bundesministerium für Bildung und Forschung, FKZ 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, and 01ER 0821) and the KfH Foundation for Preventive Medicine (Kuratorium für Heimdialyse und Nierentransplantation e.V. Stiftung Präventivmedizin) and corporate sponsors (www.gckd.org). suPAR analyses were funded by the Renal Center Heidelberg, Germany. SSH is supported by NHLBI R01HL153384, NIDDK R01DK128012 and the Gilead Sciences Research Scholar Program. US was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (grant 01ZX1912B).
Author Contributions
CS and MZ designed the study; CS, SMK, and JN analyzed the data; SSH, CS, SMK, and JN drafted the manuscript; SSH, CN, AK, JR, KUE, MS, MZ, and US revised the manuscript; all authors approved the final manuscript.
Footnotes
STROBE Statement.
Contributor Information
Salim S. Hayek, Email: shayek@med.umich.edu.
German Chronic Kidney Disease Investigators:
Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, Detlef Kraska, André Reis, Arif B. Ekici, Susanne Becker, Ulrike Alberth-Schmidt, Sabine Marschall, Eugenia Schefler, Anke Weigel, Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Kai Schmidt-Ott, Martin Zeier, Claudia Sommerer, Mehtap Aykac, Gunter Wolf, Martin Busch, Rainer Paul, Thomas Sitter, Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
Appendix
List of the German Chronic Kidney Disease Investigators
University of Erlangen-Nürnberg: Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, Detlef Kraska, André Reis, Arif B. Ekici, Susanne Becker, Ulrike Alberth-Schmidt, Sabine Marschall, Eugenia Schefler, and Anke Weigel.
University of Freiburg: Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, and Ursula Reinhard.
RWTH Aachen University: Jürgen Floege and Turgay Saritas.
Charité, University Medicine Berlin: Elke Schaeffner, Seema Baid-Agrawal, and Kerstin Theisen.
Hannover Medical School: Kai Schmidt-Ott.
University Hospital, Renal Center, Heidelberg: Martin Zeier, Claudia Sommerer, and Mehtap Aykac.
University Hospital Jena: Gunter Wolf, Martin Busch, and Rainer Paul.
Ludwig-Maximilians University of München: Thomas Sitter.
University of Würzburg: Christoph Wanner, Vera Krane, Antje Börner-Klein, and Britta Bauer.
Medical University of Innsbruck, Division of Genetic Epidemiology: Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, and Hansi Weissensteiner.
University of Regensburg, Institute of Functional Genomics: Peter Oefner and Wolfram Gronwald.
Department of Medical Biometry, Informatics and Epidemiology (IMBIE), University Hospital of Bonn: Matthias Schmid and Jennifer Nadal.
Supplementary Material
STROBE Statement.
References
- 1.van Domburg R.T., Foley D.P., de Jaegere P.P., et al. Long term outcome after coronary stent implantation: a 10 year single centre experience of 1000 patients. Heart. 1999;82(suppl 2):II27–II34. doi: 10.1136/hrt.82.2008.ii27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salsali A., Kim G., Woerle H.J., Broedl U.C., Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes Metab. 2016;18:1034–1040. doi: 10.1111/dom.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marx N., McGuire D.K., Perkovic V., et al. Composite primary end points in cardiovascular outcomes trials involving Type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40:1144–1151. doi: 10.2337/dc17-0068. [DOI] [PubMed] [Google Scholar]
- 4.Saran R., Robinson B., Abbott K.C., et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3):A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoccali C., Vanholder R., Massy Z.A., et al. The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 6.Wei C., El Hindi S., Li J., et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei C., Trachtman H., Li J., et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051–2059. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahm E., Wei C., Fernandez I., et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23:100–106. doi: 10.1038/nm.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayek S.S., Ko Y.A., Awad M., et al. Cardiovascular disease biomarkers and suPAR in predicting decline in renal function: a prospective cohort study. Kidney Int Rep. 2017;2:425–432. doi: 10.1016/j.ekir.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayek S.S., Koh K.H., Grams M.E., et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23:945–953. doi: 10.1038/nm.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayek S.S., Landsittel D.P., Wei C., et al. Soluble urokinase plasminogen activator receptor and decline in kidney function in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2019;30:1305–1313. doi: 10.1681/ASN.2018121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayek S.S., Sever S., Ko Y.A., et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo S., Coresh J., Tin A., et al. Soluble urokinase-type plasminogen activator receptor in black Americans with CKD. Clin J Am Soc Nephrol. 2018;13:1013–1021. doi: 10.2215/CJN.13631217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer F., Trachtman H., Wuhl E., et al. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2017.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei C., Li J., Adair B.D., et al. uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest. 2019;129:1946–1959. doi: 10.1172/JCI124793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayek S.S., Leaf D.E., Samman Tahhan A., et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382:416–426. doi: 10.1056/NEJMoa1911481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eugen-Olsen J., Andersen O., Linneberg A., et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 18.Persson M., Engstrom G., Bjorkbacka H., Hedblad B. Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD. Results from the Malmo diet and cancer study. Atherosclerosis. 2012;220:502–505. doi: 10.1016/j.atherosclerosis.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Botha S., Fourie C.M., Schutte R., Eugen-Olsen J., Pretorius R., Schutte A.E. Soluble urokinase plasminogen activator receptor as a prognostic marker of all-cause and cardiovascular mortality in a black population. Int J Cardiol. 2015;184:631–636. doi: 10.1016/j.ijcard.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Meijers B., Poesen R., Claes K., et al. Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int. 2015;87:210–216. doi: 10.1038/ki.2014.197. [DOI] [PubMed] [Google Scholar]
- 21.Eapen D.J., Manocha P., Ghasemzadeh N., et al. Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller L., Stojkovic S., Richter B., et al. Soluble urokinase-type plasminogen activator receptor improves risk prediction in patients with chronic heart failure. JACC Heart Fail. 2017;5:268–277. doi: 10.1016/j.jchf.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Samman Tahhan A., Hayek S.S., Sandesara P., et al. Circulating soluble urokinase plasminogen activator receptor levels and peripheral arterial disease outcomes. Atherosclerosis. 2017;264:108–114. doi: 10.1016/j.atherosclerosis.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommerer C., Zeier M., Morath C., et al. Soluble urokinase plasminogen activation receptor and long-term outcomes in persons undergoing coronary angiography. Sci Rep. 2019;9:475. doi: 10.1038/s41598-018-36960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H., Izumiya Y., Shibata A., et al. Soluble urokinase-type plasminogen activator receptor represents exercise tolerance and predicts adverse cardiac events in patients with heart failure. Heart Vessels. 2019;35:681–688. doi: 10.1007/s00380-019-01538-3. [DOI] [PubMed] [Google Scholar]
- 26.Frary C.E., Blicher M.K., Olesen T.B., et al. Circulating biomarkers for long-term cardiovascular risk stratification in apparently healthy individuals from the Monica 10 cohort. Eur J Prev Cardiol. 2019;27:570–578. doi: 10.1177/2047487319885457. [DOI] [PubMed] [Google Scholar]
- 27.Mirna M., Topf A., Wernly B., et al. Novel biomarkers in patients with chronic kidney disease: an analysis of patients enrolled in the GCKD-study. J Clin Med. 2020;9:886. doi: 10.3390/jcm9030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steins M.B., Padro T., Schwaenen C., et al. Overexpression of urokinase receptor and cell surface urokinase-type plasminogen activator in the human vessel wall with different types of atherosclerotic lesions. Blood Coagul Fibrinolysis. 2004;15:383–391. doi: 10.1097/01.mbc.0000114441.59147.56. [DOI] [PubMed] [Google Scholar]
- 29.Sehestedt T., Lyngbaek S., Eugen-Olsen J., et al. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216:237–243. doi: 10.1016/j.atherosclerosis.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Edsfeldt A., Nitulescu M., Grufman H., et al. Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke. 2012;43:3305–3312. doi: 10.1161/STROKEAHA.112.664094. [DOI] [PubMed] [Google Scholar]
- 31.Drechsler C., Hayek S.S., Wei C., et al. Soluble urokinase plasminogen activator receptor and outcomes in patients with diabetes on hemodialysis. Clin J Am Soc Nephrol. 2017;12:1265–1273. doi: 10.2215/CJN.10881016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hindy G., Tyrrell D.J., Vasbinder A., et al. Clinical, genetic, and experimental increase in soluble urokinase plasminogen activator receptor levels promotes atherosclerosis. J Clin Invest. 2022;132 doi: 10.1172/JCI158788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckardt K.U., Barthlein B., Baid-Agrawal S., et al. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27:1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 34.Titze S., Schmid M., Kottgen A., et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30:441–451. doi: 10.1093/ndt/gfu294. [DOI] [PubMed] [Google Scholar]
- 35.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riisbro R., Christensen I.J., Hogdall C., Brunner N., Hogdall E. Soluble urokinase plasminogen activator receptor measurements: influence of sample handling. Int J Biol Markers. 2001;16:233–239. doi: 10.1177/172460080101600402. [DOI] [PubMed] [Google Scholar]
- 37.Valdivielso J.M., Rodriguez-Puyol D., Pascual J., et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39:1938–1966. doi: 10.1161/ATVBAHA.119.312705. [DOI] [PubMed] [Google Scholar]
- 38.Jankowski J., Floege J., Fliser D., Bohm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Lullo L., House A., Gorini A., Santoboni A., Russo D., Ronco C. Chronic kidney disease and cardiovascular complications. Heart Fail Rev. 2015;20:259–272. doi: 10.1007/s10741-014-9460-9. [DOI] [PubMed] [Google Scholar]
- 40.Reiss A.B., Miyawaki N., Moon J., et al. CKD, arterial calcification, atherosclerosis and bone health: inter-relationships and controversies. Atherosclerosis. 2018;278:49–59. doi: 10.1016/j.atherosclerosis.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 41.Speer T., Dimmeler S., Schunk S.J., Fliser D., Ridker P.M. Targeting innate immunity-driven inflammation in CKD and cardiovascular disease. Nat Rev Nephrol. 2022;18:762–778. doi: 10.1038/s41581-022-00621-9. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen L.J.H., Petersen J.E.V., Eugen-Olsen J. Soluble urokinase plasminogen activator receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.780641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arbel Y., Strauss B.H. suPAR: a cardiac biomarker with a future? Can J Cardiol. 2015;31:1223–1224. doi: 10.1016/j.cjca.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Lyngbaek S., Marott J.L., Sehestedt T., et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904–2911. doi: 10.1016/j.ijcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 45.Shuai T., Yan P., Xiong H., et al. Association between soluble urokinase-type plasminogen activator receptor levels and chronic kidney disease: a systematic review and meta-analysis. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6927456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen M.H., Gerke O., Eugen-Olsen J., et al. Soluble urokinase plasminogen activator receptor is in contrast to high-sensitive C-reactive-protein associated with coronary artery calcifications in healthy middle-aged subjects. Atherosclerosis. 2014;237:60–66. doi: 10.1016/j.atherosclerosis.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 47.Chew-Harris J., Appleby S., Richards A.M., Troughton R.W., Pemberton C.J. Analytical, biochemical and clearance considerations of soluble urokinase plasminogen activator receptor (suPAR) in healthy individuals. Clin Biochem. 2019;69:36–44. doi: 10.1016/j.clinbiochem.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Winnicki W., Sunder-Plassmann G., Sengolge G., et al. Diagnostic and prognostic value of soluble urokinase-type plasminogen activator receptor (suPAR) in focal segmental glomerulosclerosis and impact of detection method. Sci Rep. 2019;9 doi: 10.1038/s41598-019-50405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasbinder A., Raffield L.M., Gao Y., et al. Assay-related differences in SuPAR levels: implications for measurement and data interpretation. J Nephrol. 2022;36:157–159. doi: 10.1007/s40620-022-01344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.