Highlights
-
•
Estimates of the percentage of cancer patient that are meeting physical activity guidelines vary widely between pedometer and self-report data.
-
•
Using either pedometer or self-report data an association is found between meeting physical activity guidelines and not experiencing severe fatigue, in cancer patients.
-
•
Associations, in cancer patients, between meeting physical activity guidelines and experiencing no quality of life issues or good sleep quality varied depending on the measure of activity used.
Keywords: Cancer survivorship, Fatigue, Physical activity, Quality of life, Sleep
Abstract
Background
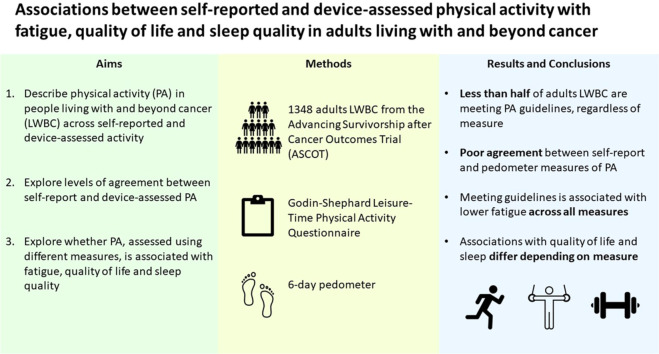
Greater physical activity is associated with improved outcomes in people living with and beyond cancer. However, most studies in exercise oncology use self-reported measures of physical activity. Few have explored agreement between self-reported and device-based measures of physical activity in people living with and beyond cancer. This study aimed to describe physical activity in adults affected by cancer across self-reported and device-assessed activity, to explore levels of agreement between these measures in terms of their utility for categorizing participants as meeting/not meeting physical activity guidelines, and to explore whether meeting guidelines is associated with fatigue, quality of life, and sleep quality.
Methods
A total of 1348 adults living with and beyond cancer from the Advancing Survivorship Cancer Outcomes Trial completed a survey assessing fatigue, quality of life, sleep quality, and physical activity. The Godin-Shephard Leisure-Time Physical Activity Questionnaire was used to calculate a Leisure Score Index (LSI) and an estimate of moderate-to-vigorous physical activity (MVPA). Average daily steps and weekly aerobic steps were derived from pedometers worn by participants.
Results
The percentage of individuals meeting physical activity guidelines was 44.3% using LSI, 49.5% using MVPA, 10.8% using average daily steps, and 28.5% using weekly aerobic steps. Agreement (Cohen's κ) between self-reported and pedometer measures ranged from 0.13 (LSI vs. average daily steps) to 0.60 (LSI vs. MVPA). After adjusting for sociodemographic and health-related covariates, meeting activity guidelines using all measures was associated with not experiencing severe fatigue (odds ratios (ORs): 1.43–1.97). Meeting guidelines using MVPA was associated with no quality-of-life issues (OR = 1.53). Meeting guidelines using both self-reported measures were associated with good sleep quality (ORs: 1.33–1.40).
Conclusion
Less than half of all adults affected by cancer are meeting physical activity guidelines, regardless of measure. Meeting guidelines is associated with lower fatigue across all measures. Associations with quality of life and sleep differ depending on measure. Future research should consider the impact of physical activity measure on findings, and where possible, use multiple measures.
Graphical abstract
1. Introduction
As early diagnosis and treatments improve, the number of people living beyond their cancer diagnosis is continually increasing.1 However, cancer and treatments can lead to long-term side effects like fatigue, impaired health-related quality of life, and sleep problems.2, 3, 4 Supportive strategies are therefore required to help with management of these. There is evidence that among adults living with and beyond cancer, higher levels of physical activity are associated with lower levels of fatigue and higher quality of life and sleep quality.5, 6, 7 The World Cancer Research Fund recommend that people living with and beyond cancer should aim for ≥150 min of moderate-to-vigorous physical activity (MVPA) per week.8
The majority of the literatures describing physical activity among people living with and beyond cancer use self-reported levels of physical activity, usually the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GSLPAQ).9, 10, 11 It is well accepted from general population data that self-reported physical activity is prone to recall bias12 and, when compared to device-based measures, can substantially overestimate the proportion of people meeting guidelines.13,14 Very few studies in exercise oncology have used device-based measures of physical activity, and the ones that do tend to have small samples. For instance, a cross-sectional study of 178 people from Alberta, Canada, affected by colon cancer found that approximately half (53.4%) of participants were achieving at least 150 min of accelerometer-assessed MVPA per week.15 Another cross-sectional study including 127 people affected by lung cancer found that participants averaged 4596 accelerometer-assessed steps per day.16
Furthermore, few studies have explored agreement between self-reported and device-based measures of physical activity in people living with and beyond cancer. Of those that have, findings have been mixed with some studies showing acceptable agreement between self-reported and device-based measures of physical activity17, 18, 19 and others showing poor agreement.20,21 However, since most studies use different measures of self-reported physical activity, findings are hard to generalize across studies. The few studies that have examined associations between the GSLPAQ and device-based physical activity have had small sample sizes (n < 200 participants) and have been conducted across a range of cancer types and ages (mean age range: 10.7–64.3 years).22, 23, 24, 25 The only one of these studies that was specifically designed to assess agreement (n = 176) found poor agreement between meeting physical activity guidelines based on self-reported data and accelerometer data in those with colon cancer (κ = 0.32).22
A small number of observational and intervention studies have examined whether device-based physical activity is associated with fatigue, quality of life, and sleep quality among people living with and beyond cancer; the results are mixed results.15,16,26, 27, 28, 29, 30, 31 A cross-sectional study of 299 women affected by breast cancer found accelerometer-assessed MVPA to be inversely associated with fatigue.27 A cross-sectional study of 178 people affected by colon cancer found accelerometer-assessed MVPA to be positively associated with health-related quality of life.15 Another cross-sectional study of 540 people affected by lung cancer found that for every one-minute increase in accelerometer-assessed MVPA, the predicted value of the 25th percentile on the 13-item Fatigue Scale32 increased by 0.16 points.16 However, in the same study,16 no association was found between physical activity and health-related quality of life. These few studies have mainly assessed associations using continuous levels of physical activity rather than by categorizing participants as meeting vs. not meeting physical activity guidelines. In addition to the possibility of poor agreement between estimates using different measures of physical activity, there could also be differential associations with fatigue, quality of life, and sleep quality.
Therefore, the aims of this study were to (1) describe the levels of physical activity reported in this sample of adults living with and beyond cancer across different measures; (2) explore the level of agreement between these measures in terms of their utility for categorizing participants as meeting vs. not meeting physical activity guidelines (150 min of MVPA per week); and (3) explore whether meeting physical activity guidelines (assessed using different measures) is associated with not experiencing severe fatigue, having no quality-of-life issues, and having good sleep quality.
2. Materials and methods
2.1. Data
This paper reports on data collected as part of the baseline assessment for the Advancing Survivorship Cancer Outcomes Trial (ASCOT) trial.33 ASCOT is a randomized controlled trial of brief lifestyle advice for cancer survivors.33 In order to assess baseline levels of activity, participants were asked to complete a self-report questionnaire (a modified version of the GSLPAQ9, 10, 11) and to wear a pedometer for 6 days. This dataset therefore provides a unique opportunity to explore self-reported and device-based physical activity and their relative associations with patient-reported outcomes of fatigue, quality of life, and sleep quality in a large sample of people living with or beyond breast, prostate, or colorectal cancer.
Participants in the ASCOT trial were recruited from 10 hospital sites across London and Essex (UK). These hospital sites were asked to send a questionnaire to patients diagnosed with breast, prostate, or colorectal cancer between 2012 and 2015. The hospitals did not always correctly identify patients, and so some participants in the trial were diagnosed outside of these dates. Patients who completed the questionnaire returned it to the research team. On the back of the questionnaire, there was the space to leave contact details if patients were interested in learning more about a trial of a lifestyle intervention. Those who expressed interest were assessed for trial eligibility. If eligible, patients could consent to participate in the trial. Eligibility criteria included being over 18 years old; reporting being diagnosed with Stages I–III breast, prostate, or colorectal cancer; and no longer receiving cancer treatments (with the exception of oral treatments taken at home). It was later discovered that 14 participants did have Stage IV cancer at diagnosis. There was no upper age limit for participation in the trial. Ethical approval for the ASCOT trial was obtained through the National Research Ethics Service Committee South Central—Oxford B (reference number 14/SC/1369), and all participants provided informed consent.
If, at the point of consent, it had been more than 8 weeks since a participant completed the initial questionnaire, a second questionnaire was sent along with the additional baseline measures, including a pedometer and log-book for participants to record when the pedometer was worn. This second questionnaire repeated the measures from the initial questionnaire, apart from those relating to demographics and clinical characteristics (e.g., quality of life measures). Participants were not required to complete all baseline assessments to be randomized. Therefore, not all participants completed the second questionnaire or provided pedometer data.
2.2. Participants
Demographic and clinical characteristics of the sample are show in Table 1. A total of 1348 patients diagnosed with breast, prostate, or colorectal cancer were recruited to the trial between August 2015 and July 2019. Participants were on average 64.3 years old and mostly female (61.4%); 54.2% had breast cancer, 27.0% had prostate cancer, and nearly 18.8% had colorectal cancer. Participants were nearly 3.5 years post-diagnosis at the time they completed the baseline questionnaire, on average.
Table 1.
Demographic and clinical characteristics of the sample.
| Characteristics | Included sample | Missing sample | Value |
|---|---|---|---|
| Demographics | |||
| Age | 1345 (99.8) | 3 (0.2) | 64.3 ± 11.4 years |
| Gender | 1348 (100.0) | 0 (0.0) | |
| Male | 520 (38.6) | ||
| Female | 828 (61.4) | ||
| Highest education | 1254 (93.0) | 94 (7.0) | |
| None | 226 (18.0) | ||
| GCSE/vocational | 412 (32.9) | ||
| A-level | 174 (13.9) | ||
| Degree or above | 442 (35.2) | ||
| Marital status | 1347 (99.9) | 1 (0.1) | |
| Married | 957 (71.0) | ||
| Unmarried | 390 (29.0) | ||
| Current employment situation | 1340 (99.4) | 8 (0.6) | |
| Employed | 510 (38.1) | ||
| Unemployed | 830 (61.9) | ||
| Ethnicity | 1342 (99.6) | 6 (0.4) | |
| White | 1242 (92.5) | ||
| Any other ethnicity | 100 (7.5) | ||
| IMD decile | 1273 (94.4) | 75 (5.6) | 6.4 ± 2.5 |
| Clinical characteristics | |||
| Cancer type | 1348 (100.0) | 0 (0.0) | |
| Breast | 730 (54.2) | ||
| Prostate | 364 (27.0) | ||
| Colorectal | 254 (18.8) | ||
| Cancer stage | 1151 (85.4) | 197 (14.6) | |
| 0 | 24 (2.1) | ||
| I | 442 (38.4) | ||
| II | 436 (37.9) | ||
| III | 235 (20.4) | ||
| IV | 14 (1.2) | ||
| Time between cancer and completing questionnaire | 1348 (100.0) | 0 (0.0) | 1254 ± 408 days |
| Treatment | 1321 (98.0) | 27 (2.0) | |
| Surgery only | 264 (20.0) | ||
| Surgery plus any other | 780 (59.0) | ||
| Any other combination | 208 (15.7) | ||
| No treatment/active surveillance | 69 (5.2) | ||
| Total number of comorbidities | 1348 (100.0) | 0 (0.0) | 1.2 ± 1.3 |
Notes: Percentages add up not to 100% due to rounding. Data are presented as n (%) or mean ± SD.
Abbreviations: GCSE = General Certificate of Secondary Education; IMD = Index of Multiple Deprivation.
2.3. Measures
2.3.1. Self-reported physical activity
Participants completed a modified version of the GSLPAQ.9, 10, 11 They were asked to report, over the past month, how many times a week on average they did each type of activity for more than 15 min during their free time. The 3 types of activity were: strenuous exercise (heart beats rapidly), moderate exercise (not exhausting), and mild exercise (minimal effort). For each type of activity, they were also asked to report the duration of each session in hours and minutes. The GSLPAQ is frequently modified to ask about the duration of sessions for each type of activity.34
To give an estimate of weekly minutes of MVPA, the frequency was multiplied by duration values for both moderate and strenuous activity. The strenuous total minutes were doubled and added to the total moderate minutes. Participants were classed as meeting the guidelines if their total weekly MVPA was 150 min or higher.
The Leisure Score Index (LSI) uses all 3 types of activity, multiplying the frequency of strenuous activities by 9, moderate by 5, and mild by 3, and then summing the scores.35 A score of 24 or above is considered to meet the World Cancer Research Fund physical activity guidelines.35
2.3.2. Pedometers measured physical activity
Participants were sent an Omron pedometer (Omron, Kyoto, Japan) with the count reader covered.36 Omron pedometer (Omron) have established validity as they provide unbiased estimates of steps at different walking and running speeds37,38 and have been shown to have an absolute percent error of less than 3% (fewer than 3 missed steps out of every 100).36 Furthermore, Omron pedometer (Omron) have established interdevice reliability as they have been shown to have coefficient of variation values of <2.1%.36 Participants were asked to wear the pedometer all day for 6 days, on their waist or in their pocket, except for when showering, bathing, swimming, or doing water sports. They were also told to remove it during contact sports. Participants were asked to complete a log-book indicating the dates when they wore the pedometer, when they put the pedometer on and took it off each day, and whether they participated in any physical activity while the pedometer was off during the days they wore it.
Data on returned pedometers were uploaded using the Omron software Bi-link gateway (Omron). This gives the number of steps taken and the number of these classified as aerobic steps (steps walked at a pace of 60 steps/min or higher for bouts of 10 min or more39) for each day.
The pedometer data were cleaned using the log-books so that (1) only days when the pedometer was worn for at least 10 h were included and (2) all minutes of physical activity that were reported to have been performed when the pedometer was not worn were included. If data were not available for 2 days, then no pedometer data were retained.40 We summed all additional activity minutes across days participants reported wearing the pedometer and divided this by the corresponding number of days to determine a daily estimate of additional activity. We used the simple conversion method suggested by Miller et al.,41 where every minute of reported physical activity when the pedometer was not worn was assumed to be equivalent to 100 steps. The average number of daily minutes was therefore multiplied by 100 and added to the average daily steps from the pedometer to give a final average daily steps variable. A cut-off of 10,000 steps was used to denote meeting physical activity guidelines.42
A value for average weekly aerobic steps was calculated by summing the average daily aerobic steps from the pedometers and the daily estimate of additional activity (minute × 100) and then multiplying this number by 7. Working on the assumption that when participants were walking at an aerobic pace, they were on average walking at a pace of 100 steps per minute, a cut-off of 15,000 aerobic steps a week was used to indicate physical activity guidelines were met. In a sub-sample of participants, the software gave aerobic walking time and number of aerobic steps, and here the average pace was approximately 100 steps per min.
2.4. Outcomes
2.4.1. Fatigue
Fatigue was measured using the Functional Assessment of Chronic Illness Therapy—Fatigue Scale (Version 4),43,44 a 13-item scale designed to measure cancer-related fatigue. Scores were dichotomized into severe fatigue (scores of 0–34) vs. not severe fatigue (scores of 35–52).45,46
2.4.2. Quality of life
Quality of life was measured using the EuroQol-5 Dimension descriptive index scale.47,48 This scale asks about problems in 5 areas: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Scores were dichotomized following the method outlined by Downing et al.49 to split participants into those who had no issues (scoring 1 “no problems” on all 5 items) vs. those who had 1 or more issues (scoring any value higher than 1 on any item).
2.4.3. Sleep quality
Sleep quality was assessed using the Pittsburgh Sleep Quality Index.50 The Pittsburgh Sleep Quality Index is composed of 19 questions that form 7 components with scores ranging from 0 to 3 for each. Two questions from Question 5 were omitted: “During the past month how often have you had trouble sleeping because you (1) cannot breathe comfortably or (2) other reason”. Question 5 is scored by adding together the responses to the 9 questions and converting the total to a component score using cut-offs (0, 1–9, 10–18, and 19–27). These have therefore been adjusted to 0, 1–7, 8–14, and 15–21 to account for the smaller number of questions within the component. Components are subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime disfunction. Component scores are combined to give a global score ranging from 0 to 21 points. A score of 0 indicates no sleep difficulties while 21 is indicative of serious difficulties in all the component areas. A Pittsburgh Sleep Quality Index global score of >5 indicates poor sleep.50
2.5. Confounders
In the initial questionnaire, participants reported on age, gender (male or female), their highest educational attainment (categorized into none, General Certificate of Secondary Education/vocational, A-level, and degree or above), marital status (dichotomized into married/living with partner vs. unmarried), current employment situation (categorized into employed or unemployed), and ethnicity (categorized into white and any other due to small numbers of participants with ethnicities other than white). Participant's postcodes were used to categorize them as to their Index of Multiple Deprivation decile based on the 2015 dataset.51 A lower decile represents a more deprived area than a higher decile.
Participants also reported on their health. They used a tick-list and a free-text box to report any comorbidities, and this was then used to calculate the total number of comorbidities reported by each participant. Participants who left this entire question blank may have missed the question, or this may reflect that they did not have any comorbidities. They reported on the treatment received for their most recent cancer, which was categorized into surgery only, surgery plus any other treatment, other treatments, and no treatment/active surveillance.
Participants also reported the date they were diagnosed with cancer, the cancer type, and stage at diagnosis. However, they were often not able to accurately report on their cancer stage at diagnosis. Participants were asked to provide consent for access to their medical records held by the National Cancer Registration and Analysis Service. If National Cancer Registration and Analysis Service data were available, this was used for their cancer diagnosis date, the site of the cancer and the cancer stage. If National Cancer Registration and Analysis Service data were not available, self-reported data were used. These variables relate to their most recent diagnosis of breast, prostate, or colorectal cancer before they were randomized into the trial. The number of days between cancer diagnosis and completion of the baseline questionnaire (in which they reported their physical activity, fatigue, quality of life, and sleep quality) was calculated and reported here.
2.6. Analysis
Statistical Package for the Social Sciences (SPSS) Version 27 (IBM Corp., Armonk, NY, USA) was used. Descriptive statistics were run for demographic, clinical characteristics, physical activity, fatigue, quality of life, and sleep quality.
To assess the level of agreement between the 4 physical activity measures (GSLPAQ LSI, GSLPAQ MVPA, pedometer average daily steps, and pedometer average weekly aerobic steps) in terms of their capacity for categorizing participants as meeting vs. not meeting the physical activity guidelines, Cohen's κ values were calculated for each pair of measures. The cut-offs used to interpret the κ values were: <0.01 (no agreement), 0.01–0.20 (none to slight), 0.21–0.40 (fair), 0.41–0.60 (moderate), 0.61–0.80 (substantial), and 0.81–1.00 (almost perfect).52
Missing value analysis found that 2.8% of 83,576 values were missing, and 49.7% of 1348 cases had at least 1 piece of missing data. Multiple imputation was conducted to impute missing data in the predictors, outcomes, and covariates, which is in line with recommendations.53 We generated 20 imputed datasets.53 The imputation was conducted twice, with similar results; therefore, the first imputed dataset was used.
The imputed dataset was used to run analyses to assess the associations between meeting physical activity guidelines and fatigue, quality of life, and sleep quality. A series of logistic regressions were run with the outcomes of not having severe fatigue, having no quality-of-life issues, and having better sleep quality. Separate regressions were run with the 4 alternative physical activity measures used to categorize participants as meeting vs. not meeting physical activity guidelines. Unadjusted regressions were run, followed by adjusted models. There are a number of confounders that could influence both physical activity and fatigue, quality of life, and sleep quality variables (listed above), and these were therefore included in the adjusted models. Cancer type was not included as this is highly correlated with gender. Due to the potential for any of the fatigue, quality of life, and sleep quality variables to be mediators between physical activity and the other (e.g., sleep could mediate between physical activity and fatigue), these were not adjusted in any of the analyses.
A sensitivity analysis was also performed. It has been suggested that because older adults have lower levels of background activity, a daily step count of 8000 steps may represent meeting physical activity guidelines in this group.42 Sensitivity analyses were therefore conducted where this number (instead of 10,000) was used to categorize participants using daily step counts.
To compare the findings to those from the imputed results, the regressions were also run among those who had complete data for each analysis.
3. Results
Well-being and levels of physical activity among the sample are shown in Table 2. Seventy-eight percent were experiencing severe fatigue, 75.6% had 1 or more quality of life problem, and 58.4% were experiencing poor sleep. The proportion classified as meeting physical activity guidelines varied widely depending on the measure used, with 10.8% meeting guidelines using average daily pedometer steps (although this increased to 23.0% when using the lower cut-off of 8000 steps), 28.5% meeting guidelines using average weekly aerobic pedometer steps, and 44.3% and 49.5% meeting guidelines when using the GSLPAQ MVPA and LSI, respectively.
Table 2.
Well-being and levels of physical activity among the sample.
| Included sample | Missing sample | Value | |
|---|---|---|---|
| Outcomes | |||
| Fatigue | 1189 (88.2) | 159 (11.8) | |
| Not severe | 261 (22.0) | ||
| Severe | 928 (78.0) | ||
| Quality of life | 1318 (97.8) | 30 (2.2) | |
| No issues | 322 (24.4) | ||
| One or more problems | 996 (75.6) | ||
| Sleep quality | 1215 (90.1) | 133 (9.9) | |
| Good | 506 (41.6) | ||
| Poor | 709 (58.4) | ||
| Physical activity | |||
| GSLPAQ Leisure Score Index | 1254 (93.0) | 94 (7.0) | 28.6 ± 21.5 |
| Meeting PA guidelines (≥24) | 621 (49.5) | ||
| Not meeting PA guidelines (<24) | 633 (50.5) | ||
| GSLPAQ MVPA (min/week) | 1235 (91.6) | 113 (8.4) | 202.4 ± 268.3 |
| Meeting PA guidelines (≥150) | 547 (44.3) | ||
| Not meeting PA guidelines (<150) | 688 (55.7) | ||
| Pedometer average daily steps | 1236 (91.7) | 112 (8.3) | 5909 ± 3287 |
| Meeting PA guidelines (≥10,000 steps) | 133 (10.8) | ||
| Not meeting PA guidelines (<10,000 steps) | 1103 (89.2) | ||
| Meeting PA guidelines (≥8000 steps) | 284 (23.0) | ||
| Not meeting PA guidelines (<8000 steps) | 952 (77.0) | ||
| Pedometer average weekly aerobic steps | 1236 (91.7) | 112 (8.3) | 11,539 ± 14,669 |
| Meeting PA guidelines (≥150,000 steps) | 352 (28.5) | ||
| Not meeting PA guidelines (<150,000 steps) | 884 (71.5) |
Note: Data are presented as number (%) or mean ± SD.
Abbreviations: GSLPAQ = Godin-Shephard Leisure-Time Physical Activity Questionnaire; MVPA = moderate-to-vigorous physical activity; PA = physical activity.
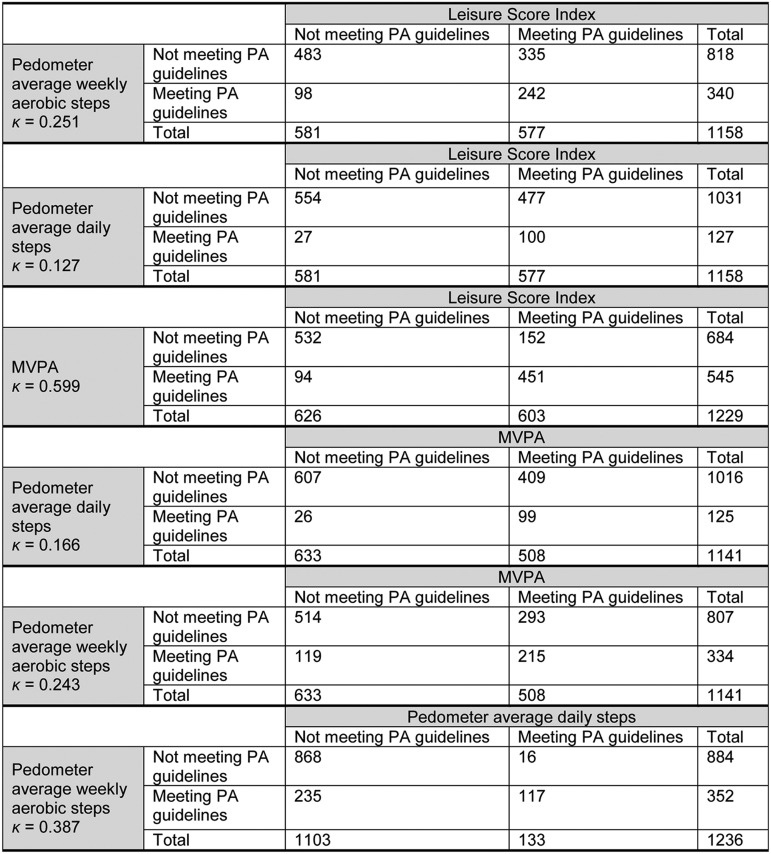
The agreement between the different measures categorization of participants as meeting the physical activity recommendations vs. not meeting them varied from a κ of 0.127 (GSLPAQ LSI vs. pedometer average daily steps) to 0.599 (GSLPAQ LSI vs. GSLPAQ MVPA) (Fig. 1).
Fig. 1.
Proportions of agreement between Leisure Score Index, MVPA, average daily steps, and weekly aerobic steps. MVPA = moderate-to-vigorous physical activity; PA = physical activity.
There was a significant association between meeting physical activity guidelines and fatigue across all measures of activity (Table 3). The effect size estimate varied depending on the measure used and ranged from a 43% to a 97% increase in the odds of not experiencing severe fatigue after adjustment for sociodemographic and health-related variables. After adjusting for potential confounders, meeting physical activity guidelines was only significantly associated with not having any quality-of-life issues when the GSLPAQ MVPA was used (Table 4). Here, meeting the guidelines increased the odds of not experiencing any issues by 53%. Lastly, meeting physical activity guidelines was associated with having good sleep quality when either of the GSLPAQ measures were used but not when pedometer values were used (Table 5). Using these measures, meeting the guidelines was associated with a 33%–40% increased odds of having good as opposed to poor sleep quality.
Table 3.
Cross-sectional associations between physical activity and fatigue in people living with and beyond breast, prostate, and colorectal cancer (n = 1348).
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| GSLPAQ Leisure Score Index | 1.71 (1.33–2.22) | <0.001 | 1.66 (1.24–2.21) | <0.001 |
| GSLPAQ MVPA | 1.84 (1.42–2.38) | <0.001 | 1.63 (1.24–2.16) | <0.001 |
| Pedometer average daily steps | 1.89 (1.15–3.11) | 0.012 | 1.97 (1.15–3.39) | 0.014 |
| Pedometer average weekly aerobic steps | 2.67 (2.31–3.09) | <0.001 | 1.43 (1.04–1.98) | 0.028 |
Note: Associations with p < 0.05 in boldface.
Adjusted for age, sex, education, ethnicity, employment status, marital status, IMD decile, number of comorbidities, time between cancer diagnosis and completing questionnaire, cancer treatment, and cancer stage.
Abbreviations: 95%CI = 95% confidence interval; GSLPAQ = Godin-Shephard Leisure-Time Physical Activity Questionnaire; IMD = Index of Multiple Deprivation; MVPA = moderate-vigorous physical activity; OR = odds ratio; PA = physical activity.
Table 4.
Cross-sectional associations between physical activity and quality of life in people living with and beyond breast, prostate, and colorectal cancer (n = 1348).
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| GSLPAQ Leisure Score Index | 1.37 (1.06–1.77) | 0.017 | 1.21 (0.92–1.60) | 0.169 |
| GSLPAQ MVPA | 1.78 (1.38–2.30) | <0.001 | 1.53 (1.17–2.01) | 0.002 |
| Pedometer average daily steps | 1.33 (0.89–1.98) | 0.163 | 1.15 (0.75–1.75) | 0.527 |
| Pedometer average weekly aerobic steps | 1.33 (1.00–1.75) | 0.047 | 1.18 (0.88–1.59) | 0.260 |
Note: Associations with p < 0.05 in boldface.
Adjusted for age, sex, education, ethnicity, employment status, marital status, IMD decile, number of comorbidities, time between cancer diagnosis and completing questionnaire, cancer treatment, and cancer stage.
Abbreviations: 95%CI = 95% confidence interval; GSLPAQ = Godin-Shephard Leisure-Time Physical Activity Questionnaire; IMD = Index of Multiple Deprivation; MVPA = moderate-vigorous physical activity; OR = odds ratio; PA = physical activity.
Table 5.
Cross-sectional associations between physical activity and sleep quality in people living with and beyond breast, prostate, and colorectal cancer (n = 1348).
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| GSLPAQ Leisure Score Index | 1.45 (1.20–1.88) | <0.001 | 1.40 (1.10–1.78) | 0.007 |
| GSLPAQ MVPA | 1.50 (1.19–1.88) | <0.001 | 1.33 (1.04–1.69) | 0.022 |
| Pedometer average daily steps | 1.11 (0.77–1.62) | 0.567 | 1.02 (0.69–1.50) | 0.938 |
| Pedometer average weekly aerobic steps | 1.25 (0.98–1.60) | 0.079 | 1.19 (0.91–1.54) | 0.200 |
Note: Associations with p < 0.05 in boldface.
Adjusted for age, sex, education, ethnicity, employment status, marital status, IMD decile, number of comorbidities, time between cancer diagnosis and completing questionnaire, cancer treatment, and cancer stage.
Abbreviations: 95%CI = 95% confidence interval; GSLPAQ = Godin-Shephard Leisure-Time Physical Activity Questionnaire; IMD = Index of Multiple Deprivation; MVPA = moderate-vigorous physical activity; OR = odds ratio; PA = physical activity.
In the completers analysis, the results were similar to the imputed dataset (Supplementary Tables 1–3). When a cut-off of 8000 rather than 10,000 average daily pedometer steps was used to classify participants as meeting vs. not meeting the guidelines, this did not change which outcomes the measure was associated with (Supplementary Table 4). It reduced the odds ratio for the association between meeting guidelines and not experiencing severe fatigue from 1.97 to 1.59.
4. Discussion
The present study found that the proportion of this sample of adults living with and beyond cancer that meet physical activity guidelines differed depending on the measure of activity used. Using Cohen's original classification of κ values, the level of agreement between self-reported and pedometer measures of physical activity was between none and fair, and the level of agreement within the 2 questionnaire and 2 pedometer measures was between fair and moderate, respectively.52 Meeting physical activity guidelines was associated with not experiencing severe fatigue across all self-reported and device-assessed measures of physical activity. Meeting activity guidelines was associated with no quality-of-life issues when self-reported MVPA was used, and with good sleep quality across both self-reported measures (but no pedometer-assessed measures).
Less than half of this sample of adults living with and beyond cancer were categorized as meeting physical activity guidelines across all measures of physical activity. This finding is in line with a large body of literature showing that most people living with and beyond cancer do not meet physical activity recommendations when physical activity is assessed using self-report.54, 55, 56 A study of 509 people affected by prostate cancer who completed the GSLPAQ found that 46% were meeting physical activity guidelines.55 Another study of 483 people affected by breast cancer from rural areas who completed the GSLPAQ found that even fewer (19.2%) were meeting physical activity guidelines.56 The lower proportion of people meeting physical activity guidelines in this study might be because the sample was from a rural population. In general, rural populations are less likely to be physically active than urban populations.57 Our study also found that fewer people were meeting physical activity guidelines when physical activity was assessed using pedometers. This finding is in line with wider literature showing that the proportion of people living with and beyond cancer meeting physical activity guidelines is lower based on accelerometer (24.3%) compared with self-reported data (37.6%).22 Given the importance of physical activity for cancer prevention and survival,58, 59, 60 it is concerning that these proportions are so low.
Using Cohen's original classification of κ values, we also found that the level of agreement between self-reported and pedometer measures of physical activity was between none and fair, and the level of agreement within the 2 questionnaire and 2 pedometer measures was fair and moderate, respectively.52 These findings are in line with other studies showing that the level of agreement between self-reported and accelerometer-based measures of physical activity is poor to fair.20, 21, 22 However, some studies have found acceptable agreement between self-reported and accelerometer-based measures of physical activity.17, 18, 19 It is important to note that pedometers are less precise measures of physical activity compared with accelerometers, as they only record total activity and not time spent at different intensities.61 However, budget did not allow collection of accelerometer data within the ASCOT trial. The poor agreement between self-reported and pedometer measures of physical activity found in our study might reflect their individual limitations. Self-report can often overestimate levels of activity, and the pedometer could underestimate them as data were included when pedometers were worn for a minimum of 10 h; hence, activity outside of these times is only captured if participants reported extra activity in their logbooks, and the adjustment for this can only be an estimate. Self-reported physical activity was included as a measure in the ASCOT trial with the intention that this could be used when pedometer data were missing at any timepoint.33 However, the lack of agreement between measures when it comes to categorizing participants as meeting vs. not meeting physical activity guidelines means that this is an invalid approach. Researchers should consider this when planning similar trials. Further large-scale work using more accurate measures of physical activity, such as accelerometers, would help us understand the proportion of adults living with and beyond cancer in the UK who meet physical activity recommendations.
The results from this study showed that meeting physical activity guidelines was associated with not experiencing severe fatigue across all self-reported and pedometer-assessed measures. These findings are in line with observational studies showing that self-reported physical activity is inversely associated with fatigue in people living with and beyond cancer,62,63 and with those showing that physical activity, assessed using accelerometers, is inversely associated with fatigue in people affected by cancer.16,26,27 There are several reasons why physical activity may be associated with fatigue in people living with and beyond cancer. First, physical activity might influence fatigue via psychosocial factors. Indeed, a longitudinal study of 1527 women affected by breast cancer found that exercise indirectly influenced fatigue at baseline and over a 6-month period via exercise self-efficacy and depression.7 Another potential mechanism linking physical activity and fatigue is changes in biological processes like inflammation. A pilot randomized controlled study of an exercise intervention with 46 people affected by breast cancer found that inflammation mediated the effect of exercise on fatigue.64 In reality a combination of factors may be at play.7 Future large-scale work across a range of cancer types is needed to clarify the biobehavioral mechanisms linking physical activity and fatigue, as this could lead to targeted interventions designed to reduce fatigue.
Our study also found that meeting physical activity guidelines was associated with quality of life for one of the self-reported measures (MVPA) and with sleep quality for both self-reported measures. Prior observational research has found that physical activity, measured using the GSLPAQ, is positively associated with quality of life65,66 and sleep quality6 in people living with and beyond cancer. The MVPA measure derived from the GSLPAQ is likely to be more accurate than the LSI because it takes account of participant reports of the length of time they participate in the different types of activity, which may explain the differing results for quality of life. It may be that adults living with and beyond cancer, who are on average an older group, do shorter bouts of activity than the general population, and this would explain why fewer appear to be meeting guidelines using the MVPA measure as opposed to the LSI.
Finally, we found no associations between meeting physical activity guidelines using pedometer measures and quality of life or sleep quality. Past research has found inconsistent results when testing for associations between quality of life and physical activity as measured by accelerometer. Some studies have found no association,16 others have found clinically important but not statistically significant improvements in some aspects of quality of life,28 and still others have uncovered statistically significant associations.15,30,67 Two intervention studies with breast cancer patients have shown that increasing physical activity can improve sleep quality.29,31 It is important to note that pedometers are not designed to capture MVPA, and although cut-offs have been suggested for the number of steps equivalent to meeting physical activity guidelines, they are only a proxy, which may explain the lack of significant results here. Again, studies using accelerometers in larger samples would be useful here to clarify the link between physical activity and quality of life and sleep quality.
The present study has several strengths. First, the study involves a large sample of people living with and beyond breast, prostate, or colorectal cancer, which are 3 of the most commonly diagnosed cancers.68 Second, this study incorporates both self-reported and device-based measures of physical activity. Third, we took into account a range of demographic and health-related confounders in the analyses. However, this study is not without limitations. First, as discussed, the cohort had agreed to take part in a trial of a lifestyle intervention, so they might not be representative of all individuals living with and beyond cancer. The study design is cross-sectional; hence, causality and the direction of causality cannot be concluded. It is possible that associations between physical activity and fatigue, quality of life, and sleep quality are bi-directional. Future work employing a longitudinal design would help clarify the direction of the associations found in the present study. Although the design is cross-sectional, the pedometer data were not always collected at the same time when the questionnaire was completed. On average, the questionnaire was completed within 13 days of the pedometer data being collected. However, given that the data used in the study was from the baseline of the trial, there is no reason to assume that either physical activity or well-being would change over this time period.
5. Conclusion
We found that most people living with and beyond cancer are not meeting physical activity recommendations, regardless of the measure of physical activity used. We also found no to slight agreement between self-reported and pedometer measures of physical activity in people living with and beyond cancer. Finally, we found that associations between physical activity with fatigue, quality of life, and sleep quality in people living with and beyond cancer differed depending on the measure of physical activity. Future work in exercise oncology should consider the impact of physical activity measure on the findings and, where possible, use multiple measures to account for their individual limitations.
Acknowledgments
Acknowledgments
We would like to acknowledge the support of the National Institute for Health and Care Research/UK Clinical Research Network in helping us recruit the participants in this study. We would also like to thank all the staff who have worked on ASCOT and, in particular, those who helped collect the pedometer data. ASCOT was funded by Cancer Research UK (grant numbers C43975/A27498 and C1418/A14133). This project involves data derived from patient-level information collected by the National Health Service (NHS) as part of the care and support of cancer patients. The data are collated, maintained, and quality assured by the National Cancer Registration and Analysis Service, which was part of Public Health England (PHE). Access to the data was facilitated by the PHE Office for Data Release prior to October 1, 2021. Data controllership now sits under NHS Digital.
Authors’ contributions
AF and RJB conceived, planned, and edited the manuscript; PL conceived, planned, drafted, and edited the manuscript; NEM planned, analysed the data, drafted, and revised the manuscript; CL planned and edited the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2023.05.001.
Supplementary materials
References
- 1.Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br J Cancer. 2012;107:1195–1202. doi: 10.1038/bjc.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower JE. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronsen S, Conway R, Lally P, et al. Determinants of sleep quality in 5835 individuals living with and beyond breast, prostate, and colorectal cancer: A cross-sectional survey. J Cancer Surviv. 2022;16:1489–1501. doi: 10.1007/s11764-021-01127-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Lee MK, Lee DH, et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: A randomized controlled trial. Support Care Cancer. 2019;27:2933–2940. doi: 10.1007/s00520-018-4588-0. [DOI] [PubMed] [Google Scholar]
- 6.Humpel N, Iverson DC. Sleep quality, fatigue and physical activity following a cancer diagnosis. Eur J Cancer Care (Engl) 2010;19:761–768. doi: 10.1111/j.1365-2354.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 7.Phillips SM, McAuley E. Physical activity and fatigue in breast cancer survivors: A panel model examining the role of self-efficacy and depression. Cancer Epidemiol Biomarkers Prev. 2013;22:773–781. doi: 10.1158/1055-9965.EPI-12-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: A global perspective. Continuous update project expert report. Available at: https://www.wcrf.org/diet-activity-and-cancer/. [accessed 19.04.2023].
- 9.Godin G. The Godin-Shephard leisure-time physical activity questionnaire. Health & Fitness Journal of Canada. 2011;4:18–22. [Google Scholar]
- 10.Godin G, Shephard R. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 11.Godin G, Shephard RJ. Godin leisure-time exercise questionnaire. Med Sci Sports Exerc. 1997;29(Suppl. 5):S36–S38. [Google Scholar]
- 12.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuoka Y, Haskell W, Vittinghoff E. New insights into discrepancies between self-reported and accelerometer-measured moderate to vigorous physical activity among women – The mPED trial. BMC Public Health. 2016;16:761. doi: 10.1186/s12889-016-3348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garriguet D, Tremblay S, Colley RC. Comparison of physical activity adult questionnaire results with accelerometer data. Health Rep. 2015;26:11–17. [PubMed] [Google Scholar]
- 15.Vallance JK, Boyle T, Courneya KS, Lynch BM. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer. 2014;120:2919–2926. doi: 10.1002/cncr.28779. [DOI] [PubMed] [Google Scholar]
- 16.D'Silva A, Gardiner PA, Boyle T, Bebb DG, Johnson ST, Vallance JK. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among lung cancer survivors: A quantile regression approach. Lung Cancer. 2018;119:78–84. doi: 10.1016/j.lungcan.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Su C-C, Lee K-D, Yeh C-H, Kao C-C, Lin C-C. Measurement of physical activity in cancer survivors: A validity study. J Cancer Surviv. 2014;8:205–212. doi: 10.1007/s11764-013-0325-3. [DOI] [PubMed] [Google Scholar]
- 18.Wagoner CW, Choi SK, Deal AM, et al. Establishing physical activity in breast cancer: Self-report versus activity tracker. Breast Cancer Res Treat. 2019;176:395–400. doi: 10.1007/s10549-019-05263-3. [DOI] [PubMed] [Google Scholar]
- 19.Amireault S, Godin G, Lacombe J, Sabiston CM. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J Cancer Surviv. 2015;9:532–540. doi: 10.1007/s11764-015-0430-6. [DOI] [PubMed] [Google Scholar]
- 20.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 21.Liu RDK, Buffart LM, Kersten MJ, et al. Psychometric properties of two physical activity questionnaires, the AQuAA and the PASE, in cancer patients. BMC Med Res Methodol. 2011;11:30. doi: 10.1186/1471-2288-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23:1121–1126. doi: 10.1007/s00520-014-2453-3. [DOI] [PubMed] [Google Scholar]
- 23.Grossman P, Deuring G, Garland SN, Campbell TS, Carlson LE. Patterns of objective physical functioning and perception of mood and fatigue in posttreatment breast cancer patients and healthy controls: An ambulatory psychophysiological investigation. Psychosom Med. 2008;70:819–828. doi: 10.1097/PSY.0b013e31818106f1. [DOI] [PubMed] [Google Scholar]
- 24.Tillmann V, Darlington AS, Eiser C, Bishop NJ, Davies HA. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res. 2002;17:1073–1080. doi: 10.1359/jbmr.2002.17.6.1073. [DOI] [PubMed] [Google Scholar]
- 25.Perna FM, Craft L, Freund KM, et al. The effect of a cognitive behavioral exercise intervention on clinical depression in a multiethnic sample of women with breast cancer: A randomized controlled trial. IJSEP. 2010;8:36–47. [Google Scholar]
- 26.Timmerman JG, Dekker-van Weering MG, Tönis TM, Hermens HJ, Vollenbroek-Hutten MM. Relationship between patterns of daily physical activity and fatigue in cancer survivors. Eur J Oncol Nurs. 2015;19:162–168. doi: 10.1016/j.ejon.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Ehlers DK, Aguiñaga S, Cosman J, Severson J, Kramer AF, McAuley E. The effects of physical activity and fatigue on cognitive performance in breast cancer survivors. Breast Cancer Res Treat. 2017;165:699–707. doi: 10.1007/s10549-017-4363-9. [DOI] [PubMed] [Google Scholar]
- 28.Gaskin CJ, Craike M, Mohebbi M, et al. Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer Causes Control. 2016;27:1093–1103. doi: 10.1007/s10552-016-0787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen NH, Vallance JK, Buman MP, et al. Effects of a wearable technology-based physical activity intervention on sleep quality in breast cancer survivors: The ACTIVATE trial. J Cancer Surviv. 2021;15:273–280. doi: 10.1007/s11764-020-00930-7. [DOI] [PubMed] [Google Scholar]
- 30.Nurnazahiah A, Shahril MR, Nor Syamimi Z, Ahmad A, Sulaiman S, Lua PL. Relationship of objectively measured physical activity and sedentary behaviour with health-related quality of life among breast cancer survivors. Health Qual Life Outcomes. 2020;18:222. doi: 10.1186/s12955-020-01478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers LQ, Courneya KS, Oster RA, et al. Physical activity and sleep quality in breast cancer survivors: A randomized trial. Med Sci Sports Exerc. 2017;49:2009–2015. doi: 10.1249/MSS.0000000000001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(Suppl. 2):S13–S19. [PubMed] [Google Scholar]
- 33.Beeken RJ, Croker H, Heinrich M, et al. Study protocol for a randomised controlled trial of brief, habit-based, lifestyle advice for cancer survivors: Exploring behavioural outcomes for the Advancing Survivorship Cancer Outcomes Trial (ASCOT) BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: A systematic review. BMC Med Res Methodol. 2015;15:60. doi: 10.1186/s12874-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amireault S, Godin G. The Godin-Shephard Leisure-Time Physical Activity Questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept Mot Skills. 2015;120:604–622. doi: 10.2466/03.27.PMS.120v19x7. [DOI] [PubMed] [Google Scholar]
- 36.Holbrook EA, Barreira TV, Kang M. Validity and reliability of Omron pedometers for prescribed and self-paced walking. Med Sci Sports Exerc. 2009;41:670–674. doi: 10.1249/MSS.0b013e3181886095. [DOI] [PubMed] [Google Scholar]
- 37.Hasson RE, Haller J, Pober DM, Staudenmayer J, Freedson PS. Validity of the Omron HJ-112 pedometer during treadmill walking. Med Sci Sports Exerc. 2009;41:805–809. doi: 10.1249/MSS.0b013e31818d9fc2. [DOI] [PubMed] [Google Scholar]
- 38.Steeves JA, Tyo BM, Connolly CP, Gregory DA, Stark NA, Bassett DR. Validity and reliability of the Omron HJ-303 tri-axial accelerometer-based pedometer. J Phys Act Health. 2011;8:1014–1020. doi: 10.1123/jpah.8.7.1014. [DOI] [PubMed] [Google Scholar]
- 39.OMRON. FAQs: Pedometers; 2010. Available at: https://omronhealthcare.com/service-and-support/faq/pedometers/. [accessed 19.04.2023].
- 40.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40:293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Miller R, Brown W, Tudor-Locke C. But what about swimming and cycling? How to “count” non-ambulatory activity when using pedometers to assess physical activity. J Phys Act Health. 2006;3:257–266. doi: 10.1123/jpah.3.3.257. [DOI] [PubMed] [Google Scholar]
- 42.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 44.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 45.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general united states population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 46.Eek D, Ivanescu C, Corredoira L, Meyers O, Cella D. Content validity and psychometric evaluation of the Functional Assessment of Chronic Illness Therapy-Fatigue scale in patients with chronic lymphocytic leukemia. J Patient Rep Outcomes. 2021;5:27. doi: 10.1186/s41687-021-00294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15:708–715. doi: 10.1016/j.jval.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 49.Downing A, Morris EJ, Richards M, et al. Health-related quality of life after colorectal cancer in England: A patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol. 2015;33:616–624. doi: 10.1200/JCO.2014.56.6539. [DOI] [PubMed] [Google Scholar]
- 50.Buysse DJ, Reynolds 3rd CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 51.Department for Communities and Local Government. The English Index of Multiple Deprivation (IMD) 2015 – Guidance. Available at: https://observatory.leeds.gov.uk/wp-content/uploads/2018/06/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf. [accessed 19.04.2023].
- 52.McHugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 53.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 55.Santa Mina D, Guglietti CL, Alibhai SMH, et al. The effect of meeting physical activity guidelines for cancer survivors on quality of life following radical prostatectomy for prostate cancer. J Cancer Surviv. 2014;8:190–198. doi: 10.1007/s11764-013-0329-z. [DOI] [PubMed] [Google Scholar]
- 56.Olson EA, Mullen SP, Rogers LQ, Courneya KS, Verhulst S, McAuley E. Meeting physical activity guidelines in rural breast cancer survivors. Am J Health Behav. 2014;38:890–899. doi: 10.5993/ajhb.38.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eberhardt MS, Pamuk ER. The importance of place of residence: Examining health in rural and nonrural areas. Am J Public Health. 2004;94:1682–1686. doi: 10.2105/ajph.94.10.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid D, Leitzmann M. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 59.Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl) 2017;95:1029–1041. doi: 10.1007/s00109-017-1558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: A systematic review. Med Sci Sports Exerc. 2019;51:1252–1261. doi: 10.1249/MSS.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowlands AV, Eston RG. Comparison of accelerometer and pedometer measures of physical activity in boys and girls, ages 8–10 years. Res Q Exerc Sport. 2005;76:251–257. doi: 10.1080/02701367.2005.10599296. [DOI] [PubMed] [Google Scholar]
- 62.Matias M, Baciarello G, Neji M, et al. Fatigue and physical activity in cancer survivors: A cross-sectional population-based study. Cancer Med. 2019;8:2535–2544. doi: 10.1002/cam4.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peddle CJ, Au H-J, Courneya KS. Associations between exercise, quality of life, and fatigue in colorectal cancer survivors. Dis Colon Rectum. 2008;51:1242–1248. doi: 10.1007/s10350-008-9324-2. [DOI] [PubMed] [Google Scholar]
- 64.Rogers LQ, Vicari S, Trammell R, et al. Biobehavioral factors mediate exercise effects on fatigue in breast cancer survivors. Med Sci Sports Exerc. 2014;46:1077–1088. doi: 10.1249/MSS.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valenti M, Porzio G, Aielli F, et al. Physical exercise and quality of life in breast cancer survivors. Int J Med Sci. 2008;5:24–28. doi: 10.7150/ijms.5.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milne HM, Gordon S, Guilfoyle A, Wallman KE, Courneya KS. Association between physical activity and quality of life among Western Australian breast cancer survivors. Psychooncology. 2007;16:1059–1068. doi: 10.1002/pon.1211. [DOI] [PubMed] [Google Scholar]
- 67.Vallance JK, Friedenreich CM, Wang Q, et al. Associations of device-measured physical activity and sedentary time with quality of life and fatigue in newly diagnosed breast cancer patients: Baseline results from the AMBER cohort study. Cancer. 2023;129:296–306. doi: 10.1002/cncr.34531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9:217–222. doi: 10.2991/jegh.k.191008.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.