Abstract
Background:
Cancer and its treatments may accelerate aging in survivors; however, research has not examined epigenetic markers of aging in longer-term breast cancer survivors. We examined whether older breast cancer survivors showed greater epigenetic aging than non-cancer controls and whether epigenetic aging related to functional outcomes.
Methods:
Non-metastatic breast cancer survivors (n=89) enrolled pre-systemic therapy and frequency-matched controls (n=101) ages 62–84 years provided two blood samples to derive epigenetic aging measures (Horvath, Extrinsic Epigenetic Age [EEA], PhenoAge, GrimAge, Dunedin Pace of Aging) and completed cognitive (FACT-Cog) and physical (SF-12) function assessments at approximately 24–36 and 60-months post-enrollment. Mixed-effects models tested survivor-control differences in epigenetic aging, adjusting for age and comorbidities; models for functional outcomes also adjusted for racial group, site, and cognitive reserve.
Results:
Survivors were 1.04–2.22 years biologically older than controls on Horvath, EEA, GrimAge, and DunedinPACE measures (p=.001 to .04) at approximately 24–36 months post-enrollment. Survivors exposed to chemotherapy were 1.97–2.71 years older (p=.001 to .04), and among this group, an older EEA related to worse self-reported cognition (p=.047) relative to controls. An older epigenetic age related to worse physical function in all women (p<.001 to .01). Survivors and controls showed similar epigenetic aging over time, but Black survivors showed accelerated aging over time relative to non-Hispanic white survivors.
Conclusion:
Older breast cancer survivors, particularly those exposed to chemotherapy, showed greater epigenetic aging than controls that may relate to worse outcomes. If replicated, measurement of biological aging could complement geriatric assessments to guide cancer care for older women.
Keywords: Breast cancer, older adults, epigenetic clock, DNA methylation, cognitive function, physical function, biological aging
Precis:
Older breast cancer survivors were biologically older than matched non-cancer controls across multiple epigenetic aging measures at 24-months or more after enrollment (which was pre-systemic therapy for survivors). Older breast cancer survivors who had received chemotherapy showed the greatest epigenetic aging, and among this group, an older epigenetic age was associated with worse self-reported cognition relative to controls.
Cancer is largely a disease of older age1–3 and 60% of women newly diagnosed with breast cancer are age 60 years and older.4,5 Providing cancer care for older women is complex because even at the same chronological age, women may vary considerably in their biological age.6 Biological aging refers to a gradual and progressive process of damage accumulation within systems due to life course experiences and exposures that leads to a loss of system reserve and increased risk of cancer and other age-related diseases, disability, and mortality.1,6–8 Cancer is a unique disease because its treatment causes damage and drives further aging.2,9–12 Assessing biological aging in cancer patients and survivors is important because it may affect their ability to manage the rigors of cancer therapy, functional outcomes, and cancer progression.2,11,13–18 However, biological aging is not always clinically apparent in oncology settings and is usually captured indirectly through measures such as comorbidities, poly-pharmacy, or functional limitations noted in geriatric assessments.19
Several markers or ‘hallmarks’ of biological aging have been identified, including telomere length, cellular senescence, and epigenetic alterations (e.g., changes in DNA methylation patterns),1 and are increasingly being investigated in clinical settings with the goal of using them to guide clinical care.6 Prior studies with breast cancer survivors have shown short-term increases in biological aging from before to up to one-year post-treatment based on epigenetic measures20 and cellular senescence marker p16INK4a.21–23 Epigenetic measures are useful because they capture biological aging over the life course, correlate with chronological age across a range of tissues and cell types, predict functional and mortality outcomes, and show feasibility for potential clinical use.24 However, previous reports have not specifically included older women or measured biological aging in a matched non-cancer control group. Further, no studies have examined epigenetic markers of aging in longer-term cancer survivors (i.e., more than one-year post-treatment) or tested whether epigenetic aging relates to common concerns in survivors, such as cognitive and physical function.
We used data from the Thinking and Living with Cancer (TLC) cohort to examine epigenetic aging in breast cancer survivors ages 60 years or older enrolled prior to systemic therapy and frequency-matched non-cancer controls. We focused on longer-term epigenetic aging starting from 24-months post-enrollment to allow for recovery from acute effects of cancer and its treatment. We tested the hypotheses that: (1) survivors would be older biologically and have faster rates of epigenetic aging than controls; (2) survivor-control differences would be largest among survivors exposed to chemotherapy; and (3) epigenetic aging would relate to worse cognitive and physical function, particularly among survivors exposed to chemotherapy. Finally, to explore how cancer may act together with differences in life course experiences to accelerate epigenetic aging, we tested whether Black survivors had different rates of epigenetic aging than white survivors. Previous research with non-cancer populations has suggested that exposure to social adversity and discrimination may contribute to observed heterogeneity in aging, including racial differences in epigenetic aging between Black and white adults.25–30 Racial disparities in breast cancer treatment-related adverse events, including increased risk for frailty and cognitive decline among Black relative to white survivors, have also been identified.31,32 In this study, we explore whether there are differences in epigenetic aging between Black and white cancer survivors. These preliminary results are intended to inform future studies testing whether biological aging markers might be clinically useful to guide treatment and survivorship care and inform the development of interventions to prevent or slow functional declines, as well as identify survivors that may be particularly vulnerable to accelerated aging following treatment, such as Black cancer survivors.
Methods
The TLC study is an ongoing national, multi-site study enrolling participants from hospitals and affiliated community practices in Washington, DC (Georgetown University), New York City (Memorial Sloan Kettering Cancer Center), New Jersey (Hackensack University Medical Center), Tampa (Moffitt Cancer Center), Los Angeles (City of Hope Comprehensive Cancer Center), and Indianapolis (Indiana University School of Medicine). All institutional review boards approved the study protocol (ClinicalTrials.gov Identifier NCT 03451383) and written informed consent was obtained from each participant. TLC study methods have been previously reported.16,33,34 This investigation involved a secondary longitudinal analysis of biological aging.
Study Population
TLC began recruitment in 2010; the study is ongoing. Eligible survivors were 60 years or older, newly diagnosed with primary non-metastatic breast cancer, English-speaking, and did not have neurological disease. Survivors were enrolled post-operatively but pre-systemic therapy. Concurrent non-cancer controls met the same eligibility and were frequency-matched to survivors by age (within 5-years), racial group, education, and site. Participants were assessed at enrollment and annually thereafter for up to 60-months.
TLC began blood collection and biobanking in 2016. This analysis includes women who had at least two blood samples collected at study assessments 24- to 60-months post-enrollment between January 1, 2016, and March 4, 2020. The latter date was selected to reflect assessments completed before the COVID-19 pandemic when the study was temporarily paused. If a participant had more than two samples available, samples with the longest time interval between them were selected. The final analytic sample included 89 survivors and 101 controls frequency-matched to survivors by age, racial group, education, site, and time between samples (Figure 1). This subsample was similar to the larger TLC cohort, except that they were younger (67.0 [SD=4.9] vs. 68.3 [SD=6.5], p=.009) and had fewer depressive symptoms (CES-D; 4.6 [SD=5.2] vs. 6.3 [SD=7.3], p=.002) at enrollment, although the latter was not a clinically meaningful difference.35
Figure 1. CONSORT diagram for the epigenetic aging sub-study within the Thinking and Living with Cancer (TLC) Study.
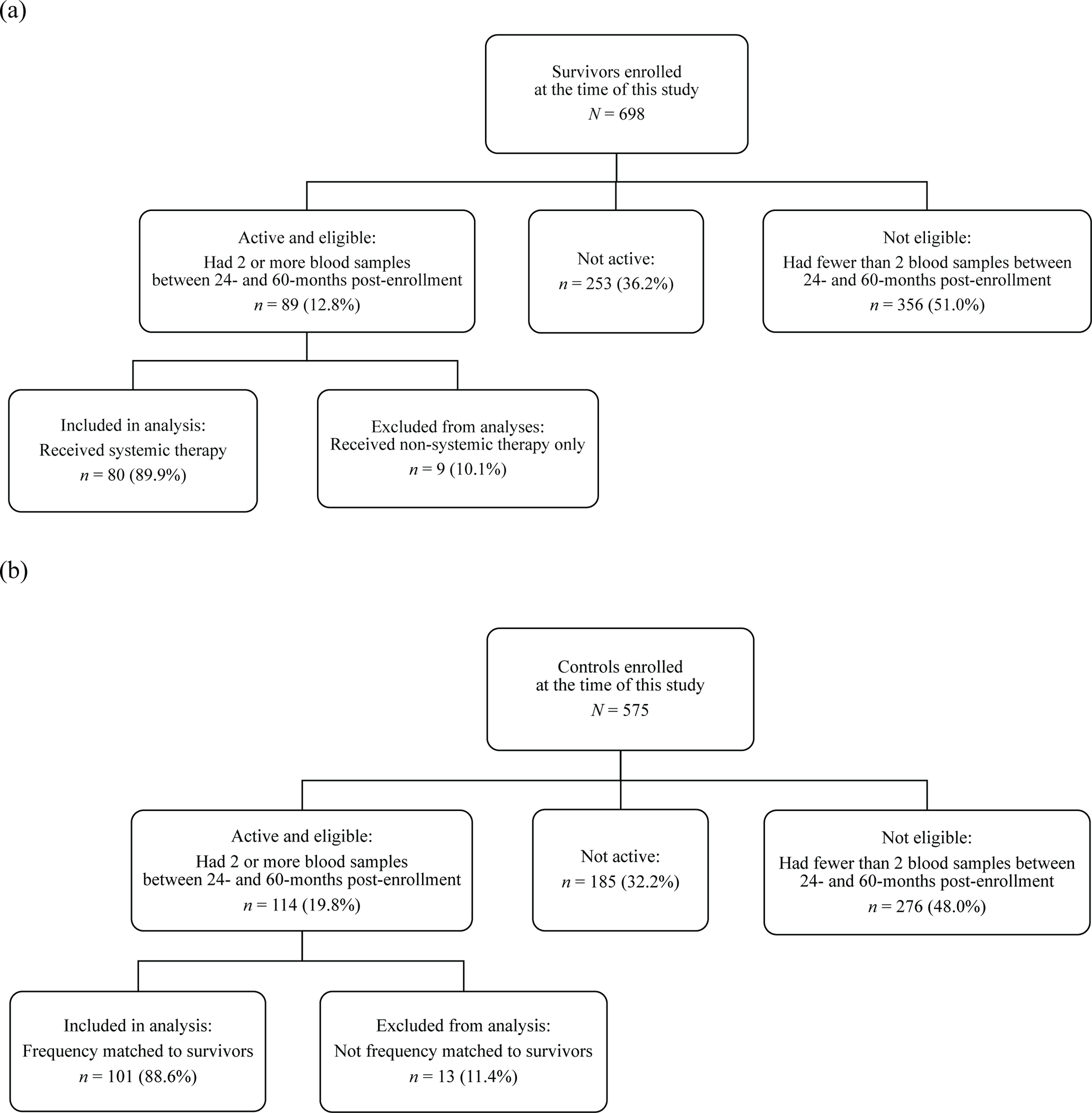
CONSORT diagram showing the sample of (a) older breast cancer survivors and (b) matched controls without cancer. This epigenetic aging study includes a subset of women who provided two blood samples between 24- and 60-months post-enrollment (which was pre-systemic therapy for survivors). Non-cancer controls were frequency-matched to survivors based on age (within 5 years), racial group, education, enrollment site, and time between blood samples.
Data Collection
Blood samples were obtained on the same day or within one week of study assessments. Whole venous blood was collected in EDTA tubes, centrifuged, and total leukocytes (buffy coat) were frozen immediately at −80°C. Samples were shipped on dry ice in a single batch to the UCLA Cousins Center for Psychoneuroimmunology for DNA extraction using the automated QIAsymphony instrument and DNA Midi kit (Qiagen) following manufacturers’ protocols. DNA was quantified using Quant-iT™ PicoGreen dsDNA kit (Qiagen).
To measure epigenetic aging, the Illumina Infinium Methylation EPIC BeadChip (Illumina, San Diego, CA) was used to quantify DNA methylation levels at more than 850,000 cytosine-phosphate-guanine (CpG) methylation sites according to the manufacturer’s protocol. All samples for a given woman were run in a single batch on the same chip. Each chip was balanced with survivors and controls, with a randomized location within the chip, to minimize laboratory variability. All testing was blinded to survivor-control status. DNA methylation data were processed following standard protocols (additional details in Supplementary Methods).36–38
Measures
We investigated several well-established, a priori-specified epigenetic estimates of biological aging, which capture slightly different aspects of the aging process (Supplemental Table 1). The most widely used approach to measuring epigenetic aging, termed the ‘epigenetic clock,’ has been proposed as a global marker of biological age because it correlates with chronological age across a range of tissues and cell types.36,39,40 We measured the Horvath, Extrinsic Epigenetic Age (EEA), Phenotypic Age (PhenoAge), and GrimAge epigenetic clocks and the Dunedin Pace of Aging methylation (DunedinPACE) measure. The Horvath clock is derived from CpG sites that are highly correlated with chronological age and is independent of cell type.36 The EEA clock also relates strongly to chronological age and upweights estimates of plasmablasts, naïve T and late-differentiated T cell proportions to account for age-related changes in cell-type composition.24,40 PhenoAge was developed to relate to phenotypic signs of aging (i.e., morbidity, mortality, circulating biomarkers of system decline),41 whereas GrimAge was developed to capture surrogate estimates of plasma markers and is a strong predictor of mortality.42 DunedinPACE was developed using a longitudinal study of biomarkers and estimates an individual’s rate of epigentic aging per chronological year.43 These measures have been linked to physical limitations,44,45 frailty,46–48 cognitive declines,49,50 and early mortality.47,51–54
At each study assessment, neuropsychological tests assessed attention, processing speed, and executive function (APE) and learning and memory (LM), as described previously,55 with lower scores indicating worse performance. The 18-item Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog)56,57 Perceived Cognitive Impairment (PCI) subscale measured self-reported cognition, with lower scores indicating greater impairment; scores that differ by 4–5 points are considered clinically meaningful. The Medical Outcomes Study Short Form-12 (MOS SF-12)58 Physical Component Summary (PCS) scale assessed physical function, with lower scores indicating worse functioning.
Chronological age at the first blood sample and comorbidities at enrollment (≤2 vs. >2) were evaluated as covariates in analyses examining survivor-control differences in epigenetic aging. Analyses of associations between epigenetic aging and cognitive and physical function considered additional covariates related to these outcomes in previous research: racial group (white vs. non-white), site, and cognitive reserve (WRAT-4 Word Reading subtest score).16
Statistical Analysis
Survivor and control groups were compared using chi-square tests and t-tests. To examine whether longitudinal changes in each epigenetic measure differed between groups, we conducted separate linear mixed-effects models that included group, time (years between blood samples, aligned at first sample), and an interaction term for group and time, adjusting for chronological age and epigenetic aging at the first blood sample and comorbidities. If an interaction was not statistically significant, it was removed from the final model and results reflect group differences in epigenetic aging at the first blood sample. To examine whether survivor-control differences in epigenetic aging differed by treatment, a second set of models compared three groups: survivors who received chemotherapy (with or without hormonal therapy), survivors who received hormonal therapy alone, and controls.
To test the hypothesis that epigenetic aging would be associated with cognitive or physical function, we conducted separate multiple regression models that included each epigenetic aging measure, group, and an interaction term for epigenetic aging and group as predictors of each functional measure, adjusting for chronological age at the first blood sample, comorbidities, racial group, site, and WRAT-4 score (the latter in cognitive models only). We tested these concurrent associations at the first blood sample only, as survivor-control differences in change in epigenetic aging over time were not observed. These analyses were then repeated to compare the three groups. If an interaction was not statistically significant, it was removed from the final model to examine associations between epigenetic aging and functional outcomes in the full sample, adjusting for group.
In secondary exploratory analyses that examined racial group differences in survivors’ epigenetic aging, linear mixed-effects models included racial group (Black vs. non-Hispanic white), time, and an interaction term for racial group and time, adjusting for chronological age and epigenetic aging at the first blood sample and comorbidities.
Statistical significance was determined using a two-sided p-value <.05. We also provided results adjusted for multiple testing using a false discovery rate of 5%.59 Analyses were conducted using SAS Version 9.4.60
Results
Participant Characteristics
Participants were 62 to 84 years old, had an average of 15 years of education, and most self-identified as non-Hispanic white (78.9%; Table 1). Due to matching, there were no survivor-control differences in sociodemographic factors. Most survivors had early-stage cancer and 32.6% received chemotherapy. Survivors were biologically older than controls on unadjusted Horvath, EEA, and DunedinPACE measures at the first blood sample (Supplemental Table 2). The majority of first blood samples were collected at 24- or 36-months post-enrollment (78.4%); most second blood samples were collected at 60-months post-enrollment (71.1%; Supplemental Table 3).
Table 1.
Enrollment characteristics of older breast cancer survivors and frequency-matched non-cancer controls1
| Survivors (n = 89) | Non-cancer controls (n = 101) | ||
|---|---|---|---|
|
|
|||
| Baseline characteristics | Mean (SD) or percent (n) | p-value | |
|
| |||
| Chronological age (years) | 70.73 (4.54) | 70.38 (5.06) | .62 |
| Race2 | |||
| American Indian/Alaska Native | 0.0 (0) | 1.0 (1) | .54 |
| Asian | 6.7 (6) | 2.0 (2) | |
| Black or African American | 7.9 (7) | 8.9 (9) | |
| Multiracial/Other | 3.4 (3) | 2.0 (2) | |
| White, Hispanic | 4.5 (4) | 5.9 (6) | |
| White, Non-Hispanic | 77.5 (69) | 80.2 (81) | |
| Education (years) | 15.66 (1.97) | 15.52 (2.48) | .67 |
| Marital status | |||
| Married or living as married | 63.6 (56) | 56.0 (56) | .28 |
| Other | 36.4 (32) | 44.0 (44) | |
| Missing | 1.1 (1) | 1.0 (1) | |
| Cognitive reserve, WRAT-4 Word Reading score | 112.03 (15.08) | 112.82 (17.76) | .74 |
| Number of comorbidities | |||
| ≤ 2 (median) | 50.6 (44) | 56.6 (56) | .41 |
| > 2 | 49.4 (43) | 43.4 (43) | |
| Missing | 2.2 (2) | 2.0 (2) | |
| AJCC stage | |||
| 0 | 15.7 (14) | ||
| I | 61.8 (55) | ||
| II | 16.9 (15) | ||
| III | 5.6 (5) | ||
| Molecular subtype | |||
| ER+ and HER2− | 75.6 (62) | ||
| HER2+ | 17.1 (14) | ||
| Triple negative | 7.3 (6) | ||
| Missing | 7.9 (7) | ||
| Surgery | |||
| Lumpectomy with radiotherapy | 43.8 (39) | ||
| Lumpectomy without radiotherapy | 12.4 (11) | ||
| Mastectomy | 43.8 (39) | ||
| Hormonal therapy regimen | |||
| Aromatase inhibitor | 89.7 (61) | ||
| Tamoxifen | 10.3 (7) | ||
| None | 23.6 (21) | ||
| Chemotherapy regimen | |||
| AC (no T) | 6.9 (2) | ||
| AC+T | 55.2 (16) | ||
| CMF | 3.4 (1) | ||
| T only | 34.5 (10) | ||
| None | 67.4 (60) | ||
Enrollment was pre-systemic therapy for survivors or study start for controls.
Racial/ethnic groups were collapsed to Black vs. non-Hispanic white for analyses.
AC = Adjuvant chemotherapy (doxorubicin and cyclophosphamide); T = Taxotere; CMF = cyclophosphamide, methotrexate, and 5-fluorouracil; p-values were derived from t-tests or chi-square tests; p-values <.05 appear in bold font.
Differences in Epigenetic Aging Between Groups
At the first blood sample, survivors were 1.04 to 2.22 years older on the Horvath, EEA, and GrimAge measures (p=.006 to .035) and had a faster DunedinPACE (p=.001) than controls, adjusting for covariates (Figure 2; Table 2). These differences were largest among survivors who received chemotherapy (with or without hormonal therapy), who were 1.97 to 2.71 years older on the Horvath, EEA, and GrimAge measures (p=.001 to .04) and had a faster DunedinPACE (p=.002) than controls. Survivors who received hormonal therapy alone were 1.61 years older on the Horvath measure (p=.04) and had a faster DunedinPACE (p=.02) than controls. However, survivors and controls aged at a similar rate over time.
Figure 2. Differences in epigenetic aging between older breast cancer survivors and non-cancer controls at the first blood sample, approximately 24- to 36-months after enrollment (which was pre-systemic therapy for survivors).
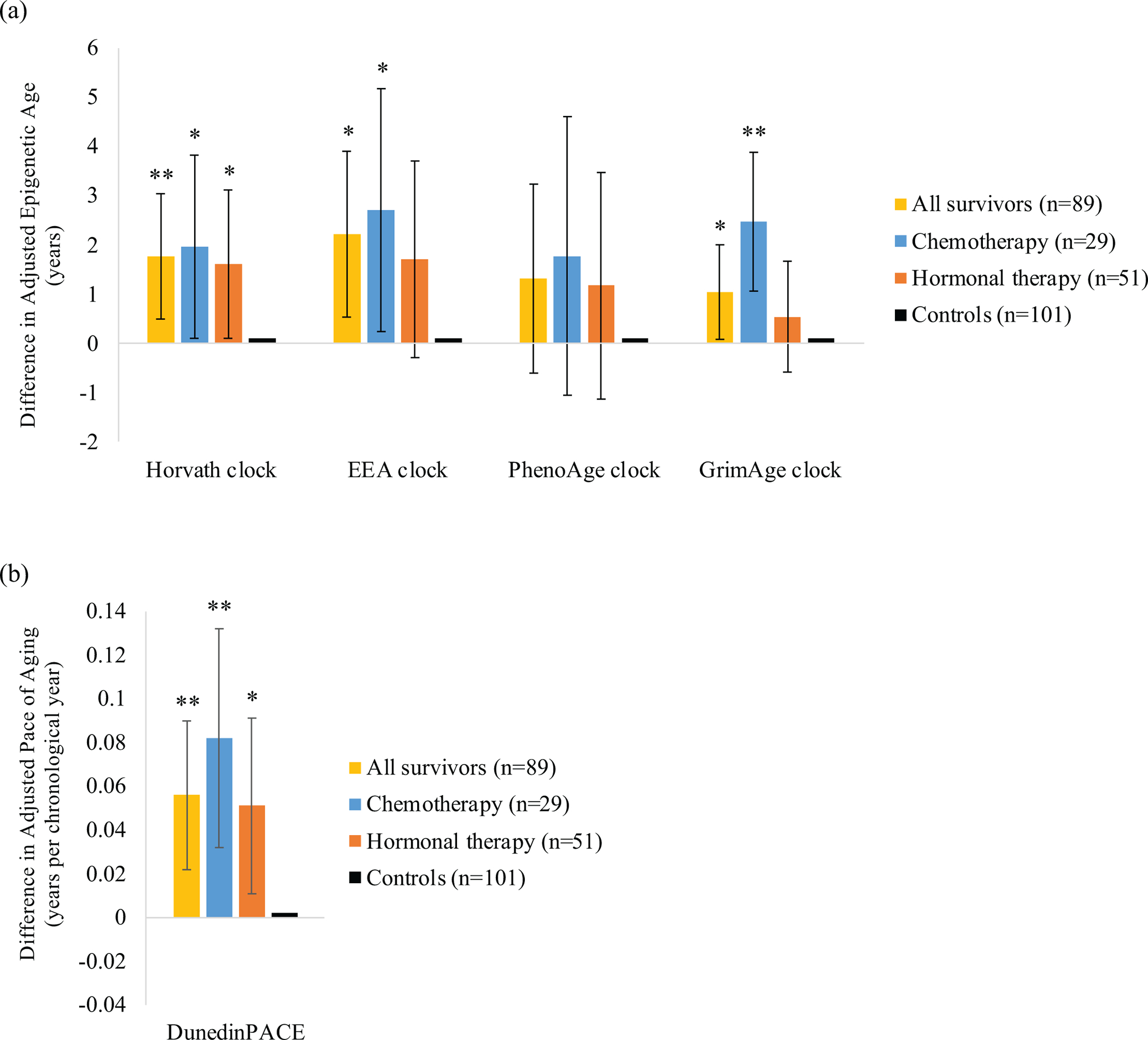
Point estimates for differences in (a) adjusted epigenetic age and (b) adjusted Dunedin Pace of Aging (DunedinPACE) at the first blood sample for all survivors (n=89), as well as survivors who received chemotherapy (without or without hormonal therapy; n=29) or hormonal therapy alone (n=51), relative to matched non-cancer controls (n=101) at the first blood sample. Models adjusted for chronological age at the first blood sample and comorbidities. Error bars represent 95% confidence intervals for each point estimate. **p<.01, *p<.05.
Table 2.
Differences in adjusted epigenetic aging between older breast cancer survivors and controls at the first blood sample, approximately 24- to 48-months post-enrollment
| Horvath clock | EEA clock | PhenoAge clock | GrimAge clock | DunedinPACE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Model | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p |
|
| |||||||||||||||
| 1. Group (n=190) | |||||||||||||||
| Survivors | 1.76 | 0.50 to 3.03 | .006 [.02] | 2.22 | 0.53 to 3.90 | .01 [.02] | 1.32 | −0.60 to 3.24 | .18 [.18] | 1.04 | 0.08 to 2.00 | .04 [.04] | 0.06 | 0.02 to 0.09 | .001 [.005] |
| Controls | (reference) | (reference) | (reference) | (reference) | (reference) | ||||||||||
| 2. Group (n=181) | |||||||||||||||
| Chemotherapy | 1.97 | 0.10 to 3.83 | .04 [.049] | 2.71 | 0.24 to 5.18 | .03 [.049] | 1.77 | −1.05 to 4.60 | .22 [.22] | 2.47 | 0.08 to 3.87 | .001 [.005] | 0.08 | 0.03 to 0.13 | .002 [.005] |
| Hormonal therapy | 1.61 | 0.10 to 3.12 | .04 [.09] | 1.71 | −0.30 to 3.71 | .095 [.16] | 1.17 | −1.13 to 3.46 | .32 [.35] | 0.54 | −0.59 to 1.67 | .35 [.35] | 0.05 | 0.01 to 0.09 | .02 [.08] |
| Controls | (reference) | (reference) | (reference) | (reference) | (reference) | ||||||||||
Note. Models adjusted for chronological age at the first blood sample and comorbidities. Given that group differences in epigenetic aging over time were not statistically significant, time and group-by-time interactions were removed from the final models, leaving group differences in epigenetic aging at the time of the first blood sample. Regression coefficients (B) for the epigenetic clock measures indicate the difference in epigenetic age (years) between each group of survivors and matched non-cancer controls. Regression coefficients for the DunedinPACE measure indicate the difference in epigenetic aging for one year of chronological age between each group of survivors and controls. Model 2 excludes 9 survivors who received non-systemic treatments (e.g., surgery, radiotherapy) only. p-values <.05 appear in bold font; a False Discovery Rate is shown in brackets.
In exploratory analyses, Black survivors showed a larger increase in epigenetic age on the Horvath (p=.03) and PhenoAge (p=.048) measures from the first to second blood sample compared to non-Hispanic white survivors (Figure 3; Supplemental Table 4).
Figure 3. Longitudinal changes in epigenetic aging between the first and second blood samples among Black or non-Hispanic white older breast cancer survivors.
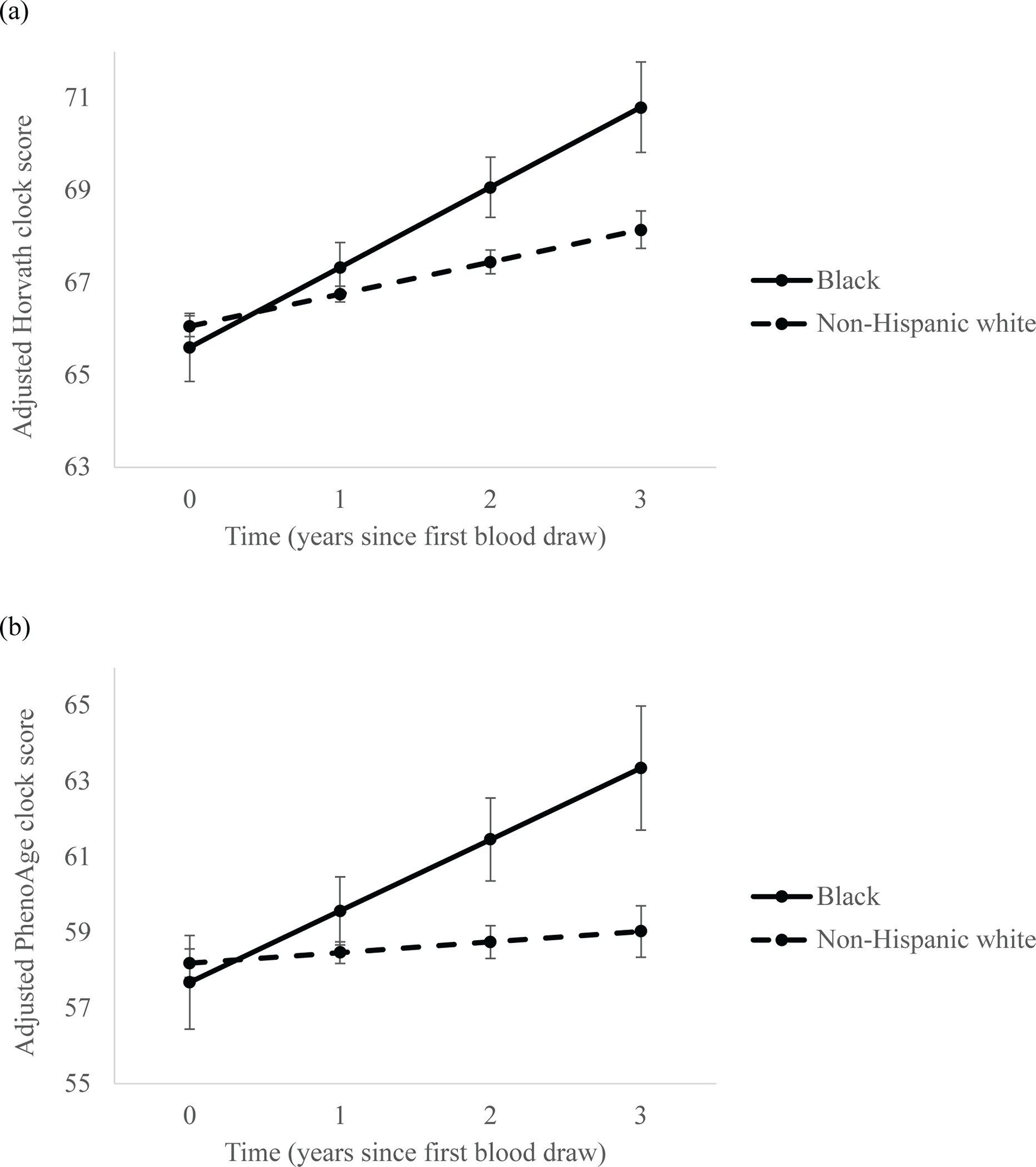
Linear mixed-effects models showing estimated longitudinal epigenetic aging from the first to second blood samples, collected between approximately 24- to 36-months and up to 60-months post-enrollment, in Black (n=7) and non-Hispanic white (n=69) breast cancer survivors based on (a) Horvath clock and (b) PhenoAge clock estimates. Models adjusted for chronological and epigenetic age at the first blood sample and comorbidities. Error bars represent 95% confidence intervals for each point estimate.
Concurrent Associations between Epigenetic Aging and Cognitive and Physical Function
Overall survivor-control differences in associations between epigenetic aging and functional measures were not observed (data not shown). In analyses that compared the three groups, among survivors who received chemotherapy, an older EEA was associated with worse self-reported cognition relative to controls (p=.047), with a similar but marginal association for GrimAge (p=.08; Figure 4; Supplemental Table 5). The other epigenetic aging measures were not associated with self-reported cognition, and none of the epigenetic measures were related to performance on neuropsychological tests. In follow-up analyses that examined these associations in all women regardless of survivor-control status, an older Horvath age, PhenoAge, and GrimAge and a faster DunedinPACE related to worse physical function (ps<.001 to .01; Supplemental Table 6).
Figure 4. Associations between adjusted Extrinsic Epigenetic Age (EEA) and self-reported cognition among older breast cancer survivors by systemic therapy modality and non-cancer controls at the first blood sample, approximately 24- to 36-months after enrollment.
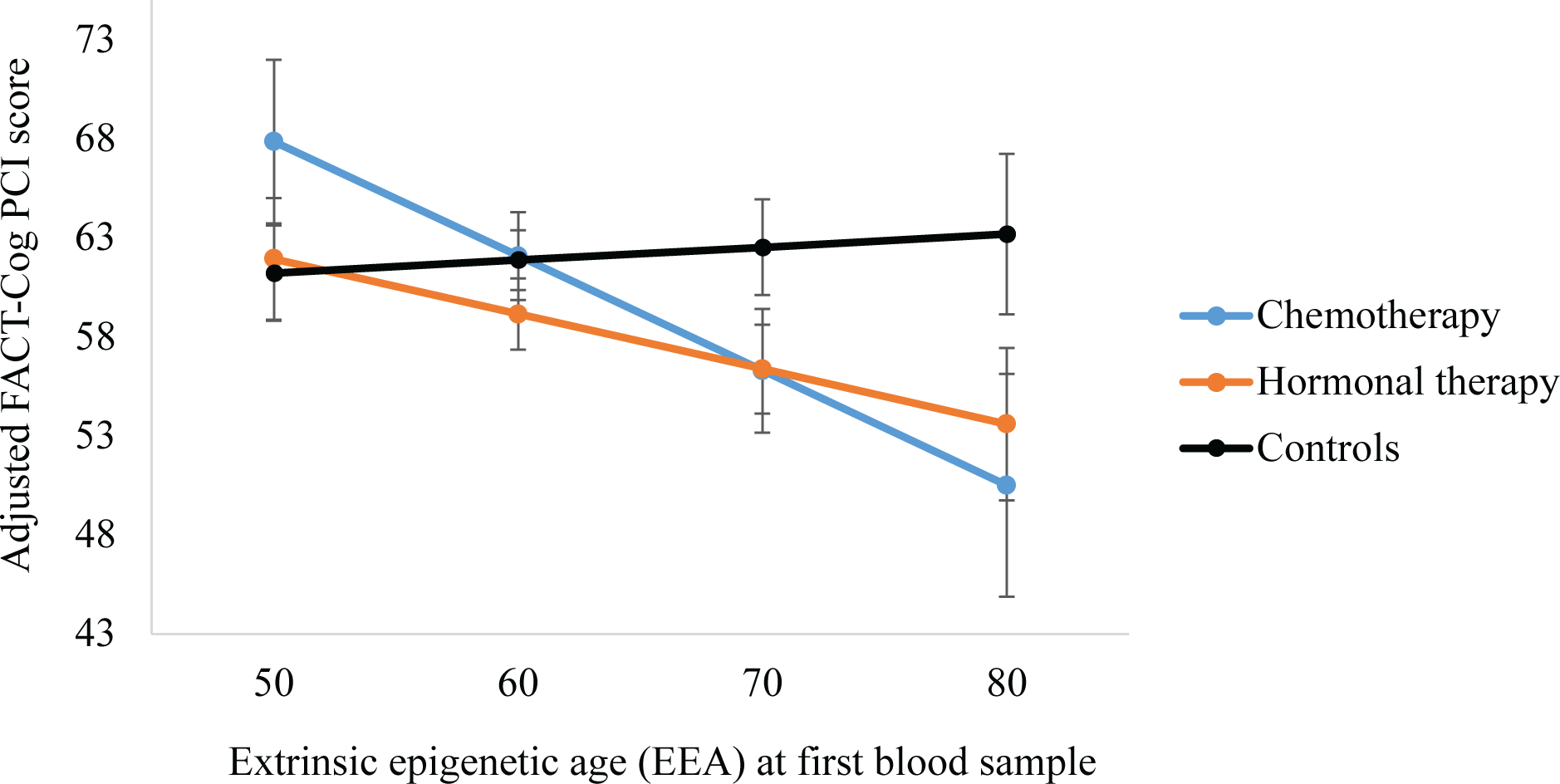
Multiple regression models showing the association between adjusted EEA clock scores and self-reported cognition (FACT-Cog PCI scores) for survivors who received chemotherapy with or without hormonal therapy (n=29) and those who received hormonal therapy alone (n=51) relative to controls (n=101,). Models adjusted for chronological age at the first blood sample, comorbidities, racial group, site, and cognitive reserve (WRAT-4 score). Error bars represent 95% confidence intervals for each point estimate.
Discussion
This preliminary report presents unique, new data comparing longer-term epigenetic aging among older breast cancer survivors to that of matched non-cancer controls. We found that survivors were older biologically than controls across multiple epigenetic aging measures at the first observed timepoint, approximately 24- to 36-months after enrollment (which was pre-systemic therapy for survivors). Survivors exposed to chemotherapy (with or without hormonal therapy) showed the largest differences, followed by those on hormonal therapy alone, relative to controls. Interestingly, survivors and controls did not differ in their rate of change in epigenetic aging (i.e., slopes) from the first to second timepoint, suggesting that they increased in parallel over time. There were, however, racial differences in epigenetic aging over time among survivors, with Black survivors showing a larger increase in epigenetic age from the first to second timepoint than non-Hispanic white survivors.
Prior studies of biological aging in breast cancer survivors have found short-term increases from before to up to one-year post-treatment, based on epigenetic measures and cellular senescence marker p16INK4a.20,21 Breast cancer survivors who received chemotherapy and/or radiotherapy also had higher DNA damage and lower telomerase (an enzyme that protects telomeres) 3–6 years after treatment relative to surgery alone,61 which was associated with worse cognitive function.62 We extend these investigations by using a chronologically older sample, including a concurrent non-cancer control group, and examining longer-term biological aging in the period 24-months or more after enrollment (which was pre-systemic therapy for survivors). We focused on this period to allow for the completion of all active therapy (surgery, radiotherapy, chemotherapy, and targeted treatments).
Together with previous studies, our results suggest that some of the biological aging observed immediately following active therapy persists for up to 60-months and is greater than would be expected in the absence of cancer. Given that we did not observe survivor-control differences in the rate of change in epigenetic aging (i.e., slopes) from the first to second observed timepoint, it is possible that the effects of cancer and its treatment on biological aging may level off after a few years. However, since most survivors in our sample were receiving five years of hormonal therapy, it will be critical to re-assess these findings in studies with follow-up beyond completion of that regimen. Additionally, given that biological aging increases the risk of cancer,54,63,64 we cannot determine whether our and others’ results reflect greater biological aging among survivors prior to diagnosis. Advancing the field of cancer and aging will require longitudinal survivor-control designs and cohorts with data prior to cancer diagnosis to more fully understand the temporal dynamics of biological aging in the cancer context.
In terms of the associations between epigenetic aging and functional measures, we found that an older epigenetic age was concurrently associated with worse self-reported physical function in all women (regardless of survivorship status) and worse cognition in survivors who received chemotherapy but not in controls. At an epigenetic age of 70, survivors had an adjusted cognition score that was more than six points lower than controls with the same epigenetic age, which is a clinically meaningful difference.16,65 Interestingly, epigenetic aging was associated with self-report but not with neuropsychological assessments of cognition in this sample, suggesting that the more global self-report measure may be more sensitive in reflecting underlying epigenetic differences between groups than objectively assessed cognitive performance, underscoring the value of including patient-reported outcome measures in research on late effects of treatment. This is consistent with our previous finding that inflammation, another hallmark of aging, related to worse self-reported cognition among survivors, especially those receiving chemotherapy.66 Our results are also consistent with prior research in non-cancer populations linking epigenetic aging to worse cognitive and physical function in older adulthood.43,44,46,48,49 However, given the small number of survivors who received chemotherapy in our sample, these findings should be replicated. It will also be useful to test whether specific chemotherapeutic agents are linked to biological aging and longer-term functional declines and to explore the additive or interactive effects of multi-modality therapy regimens, as research has also linked breast cancer radiotherapy to biological aging 3–6 years after treatment.61
Although exploratory, we found that Black survivors showed accelerated epigenetic aging on two measures from the first to second observed timepoint relative to non-Hispanic white survivors, after adjusting for covariates. Given the small number of Black survivors in our sample, future research should address this question in a larger sample and test whether differences contribute to disparities in functional outcomes. Beyond racial group identity, future investigations should also consider life course experiences such as exposure to stressful or traumatic life events and racial discrimination prior to and after cancer diagnosis, which previous studies have linked to accelerated epigenetic aging67,68 and other biological aging markers.69,70
Our results should be considered in light of study limitations. Although TLC is a relatively large study, the protocol for blood collection was a recent addition, limiting the sample size for this analysis. The subsample included in this preliminary report was generally comparable to the larger study cohort and the analysis yielded statistically significant effects, even with the smaller sample. Also, TLC participants had a high level of education and were in relatively good health, reducing generalizability. However, since the characteristics of our sample are associated with less biological aging in the general population,28,71,72 our results may have underestimated epigenetic aging and/or biased survivor-control differences toward the null. Finally, given that approximately one-third of older survivors in our sample received chemotherapy, we were unable to test the impact of different treatment regimens. This will be an important direction for subsequent studies, especially as the chemotherapy and hormonal therapy landscape continues to evolve.
This is the first investigation of longer-term biological aging in older breast cancer survivors and matched non-cancer controls. We found that survivors showed greater biological aging, as measured by epigenetic assays, than controls. Older breast cancer survivors who had received chemotherapy showed the greatest epigenetic aging, and among this group, an older epigenetic age was associated with worse self-reported cognition. The exploratory finding of racial group differences in the rate of change of biological aging is thought provoking and suggests that studying the intersectionality of aging and cancer may provide insights into causes of cancer disparities. Given emerging evidence in humans and mice suggesting that epigenetic aging may be modifiable by behavioral and pharmacological interventions,73–79 these preliminary results can also inform future studies testing whether biological aging markers might be clinically useful as early indicators of risk that can be used to tailor survivorship care, including interventions to maintain health and prevent or slow functional declines.
Supplementary Material
Acknowledgements:
We would like to thank the participants in the Thinking and Living with Cancer (TLC) study for sharing their time and experiences; without their generosity this study would not have been possible. We are also indebted to Sherri Stahl, Naomi Greenwood, Margery London, and Sue Winarsky, who serve as patient advocates from the Georgetown Breast Cancer Advocates, for their insights and suggestions on study design and methods to recruit and retain participants. We thank the TLC study staff who contributed by ascertaining, enrolling, and interviewing participants.
Role of the funders:
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health through Grant R35CA197289 to J.S.M. The research was also supported in part by the National Cancer Institute through grant numbers R01CA129769 (J.S.M.), P30CA51008 (Georgetown-Lombardi Comprehensive Cancer Center for Shared Resources), K01CA212056 (T.N.B.), K08CA241337 (K.V.D.), R01CA172119 (T.A.A.), U54CA137788 (T.A.A.), P30CA008748 (T.A.A), and R01CA244673 (B.C.M.); National Institute on Aging at the National Institutes of Health through grant numbers R56AG068086 (J.E.C. and J.S.M) R01AG068193 (J.S.M. and A.J.S.), R01CA237535 (J.E.C.), K01AG065485 (K.E.R.), P30AG028716 (H.J.C.), P30AG010133 (A.J.S.), and R01CA261793 (S.K.P.) and UCLA Cousins Center for Psychoneuroimmunology (K.E.R. and J.E.C.).
Footnotes
Conflict of interest disclosure
M.E. served as a consultant for Aileron and Alnylam and received honoraria from OncLive. The authors have declared no other conflicts of interest.
Ethics approval
All institutional review boards approved the study protocol, and this research was conducted in accordance with ethical standards, including the Declaration of Helsinki and the US Federal Policy for the Protection of Human Subjects.
Patient consent
Written informed consent was obtained from each participant prior to their inclusion in the study.
Prior presentations: A portion of these findings were presented at the Annual Meeting of the Gerontological Society of America (GSA) in Indianapolis, Indiana on November 4, 2022, and the American Association for Cancer Research (AACR) Special Conference on Cancer and Aging in San Diego, CA on November 19, 2022.
Data Availability
The data collected for the Thinking and Living with Cancer (TLC) study used in this publication were supported by funding from the National Institutes of Health. The data are available for sharing under NIH-compliant TLC study agreements and FAIR principles (Findability, Accessibility, Interoperability, Reproducibility) for data access. Data access is provided via email requests to the study Principal Investigators and follows the TLC protocol. The SAS code and data for the analyses included in this paper are available upon request within the constraints of the TLC IRB requirements.
References
- 1.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann N Y Acad Sci. 2016;1386(1):45–68. doi: 10.1111/NYAS.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cespedes Feliciano EM, Hohensee C, Rosko AE, et al. Association of Prediagnostic Frailty, Change in Frailty Status, and Mortality After Cancer Diagnosis in the Women’s Health Initiative. JAMA Netw Open. 2020;3(9):e2016747–e2016747. doi: 10.1001/JAMANETWORKOPEN.2020.16747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surveillance Research Program. SEER*Explorer: An interactive website for SEER cancer statistics. National Cancer Institute. Published July 21, 2022. Accessed July 20, 2022. https://seer.cancer.gov/statistics-network/explorer/ [Google Scholar]
- 6.Mandelblatt JS, Ahles TA, Lippman ME, et al. Applying a Life Course Biological Age Framework to Improving the Care of Individuals With Adult Cancers: Review and Research Recommendations. JAMA Oncol. 2021;7(11):1692–1699. doi: 10.1001/JAMAONCOL.2021.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy BJ. Aging and cancer. https://doi.org/101200/JCO19886121903. 2016;6(12):1903–1911. doi: 10.1200/JCO.1988.6.12.1903 [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: A quest. Aging Cell. 2020;19(2). doi: 10.1111/acel.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soria-Valles C, López-Soto A, Osorio FG, López-Otín C. Immune and inflammatory responses to DNA damage in cancer and aging. Mech Ageing Dev. 2017;165:10–16. doi: 10.1016/J.MAD.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 10.Muhandiramge J, Orchard S, Haydon A, Zalcberg J. The acceleration of ageing in older patients with cancer. J Geriatr Oncol. 2021;12(3):343–351. doi: 10.1016/J.JGO.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 11.Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2(5):e000250. doi: 10.1136/ESMOOPEN-2017-000250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Weiner LS, Hartman SJ, et al. Breast cancer treatment and its effects on aging. J Geriatr Oncol. 2019;10(2):346. doi: 10.1016/J.JGO.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll JE, Bower JE, Ganz PA. Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care. Nature Reviews Clinical Oncology 2021. Published online December 6, 2021:1–15. doi: 10.1038/s41571-021-00580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfano CM, Peng J, Andridge RR, et al. Inflammatory Cytokines and Comorbidity Development in Breast Cancer Survivors Versus Noncancer Controls: Evidence for Accelerated Aging? Journal of Clinical Oncology. 2017;35(2):149. doi: 10.1200/JCO.2016.67.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness KK, Wogksch MD. Frailty and aging in cancer survivors. Translational Research. 2020;221:65–82. doi: 10.1016/J.TRSL.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandelblatt JS, Small BJ, Luta G, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. Journal of Clinical Oncology. 2018;36(32):3211–3222. doi: 10.1200/JCO.18.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahles TA, Root JC, Ryan EL. Cancer- and Cancer Treatment–Associated Cognitive Change: An Update on the State of the Science. Journal of Clinical Oncology. 2012;30(30):3675. doi: 10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guida JL, Ahles TA, Belsky D, et al. Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. 2019;111(12):djz136. doi: 10.1093/jnci/djz136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkhus L, Benth JŠ, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. British Journal of Cancer 2017 117:4. 2017;117(4):470–477. doi: 10.1038/bjc.2017.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. npj Breast Cancer 2020 6:1 2020;6(1):1–5. doi: 10.1038/s41523-020-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4). doi: 10.1093/jnci/dju057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shachar SS, Deal AM, Reeder-Hayes KE, et al. Effects of Breast Cancer Adjuvant Chemotherapy Regimens on Expression of the Aging Biomarker, p16INK4a. JNCI Cancer Spectr. 2020;4(6). doi: 10.1093/JNCICS/PKAA082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shachar SS, Deal AM, Mitin N, et al. Changes in p16INK4a (p16) expression, a biomarker of aging, in peripheral blood T-cells (PBTC) in patients receiving anthracycline (A) vs non-anthracycline (NoA) chemotherapy (CRx) for early-stage breast cancer (EBC). https://doi.org/101200/JCO20173515_suppl10060. 2017;35(15_suppl):10060–10060. doi: 10.1200/JCO.2017.35.15_SUPPL.10060 [DOI] [Google Scholar]
- 24.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 25.Brody GH, Miller GE, Yu T, Beach SRH, Chen E. Supportive Family Environments Ameliorate the Link Between Racial Discrimination and Epigenetic Aging: A Replication Across Two Longitudinal Cohorts. Psychol Sci. 2016;27(4):530–541. doi: 10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Chen BH, Assimes TL, Ferrucci L, Horvath S, Levine ME. The role of epigenetic aging in education and racial/ethnic mortality disparities among older U.S. Women. Psychoneuroendocrinology. 2019;104:18–24. doi: 10.1016/j.psyneuen.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamlat EJ, Adler NE, Laraia B, et al. Association of subjective social status with epigenetic aging among Black and White women. Psychoneuroendocrinology. 2022;141:105748. doi: 10.1016/J.PSYNEUEN.2022.105748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crimmins EM, Thyagarajan B, Levine ME, Weir DR, Faul J. Associations of Age, Sex, Race/Ethnicity, and Education With 13 Epigenetic Clocks in a Nationally Representative U.S. Sample: The Health and Retirement Study. The Journals of Gerontology: Series A. 2021;76(6):1117–1123. doi: 10.1093/GERONA/GLAB016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons RL, Lei MK, Klopack E, Beach SRH, Gibbons FX, Philibert RA. The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Soc Sci Med. 2021;282:113169. doi: 10.1016/J.SOCSCIMED.2020.113169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons RL, Lei MK, Beach SRH, et al. Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of black women. Soc Sci Med. 2016;150:192–200. doi: 10.1016/j.socscimed.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabellini N, Cullen J, Cao L, et al. Racial disparities in breast cancer treatment patterns and treatment related adverse events. Scientific Reports 2023 13:1. 2023;13(1):1–10. doi: 10.1038/s41598-023-27578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mandelblatt JS, Ruterbusch JJ, Thompson HS, et al. Association between major discrimination and deficit accumulation in African American cancer survivors: The Detroit Research on Cancer Survivors Study. Cancer. Published online March 20, 2023. doi: 10.1002/CNCR.34673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive Impairment in Older Patients With Breast Cancer Before Systemic Therapy: Is There an Interaction Between Cancer and Comorbidity? Journal of Clinical Oncology. 2014;32(18):1909. doi: 10.1200/JCO.2013.54.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelblatt JS, Zhai W, Ahn J, et al. Symptom burden among older breast cancer survivors: The Thinking and Living With Cancer (TLC) study. Cancer. 2020;126(6):1183–1192. doi: 10.1002/CNCR.32663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busch AM, Wagener TL, Gregor KL, Ring KT, Borrelli B. Utilizing reliable and clinically significant change criteria to assess for the development of depression during smoking cessation treatment: The importance of tracking idiographic change. Addictive Behaviors. 2011;36(12):1228–1232. doi: 10.1016/j.addbeh.2011.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath S DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Triche TJ, Weisenberger DJ, van den Berg D, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013;41(7):e90–e90. doi: 10.1093/NAR/GKT090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, A DNA methylation biomarker of the Pace of Aging. Elife. 2022;11. doi: 10.7554/eLife.73420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bocklandt S, Lin W, Sehl ME, et al. Epigenetic predictor of age. PLoS One. 2011;6(6):e14821. doi: 10.1371/journal.pone.0014821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannum G, Guinney J, Zhao L, et al. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol Cell. 2013;49(2):359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11(2):303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:1–56. doi: 10.7554/eLife.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maddock J, Castillo-Fernandez J, Wong A, et al. DNA methylation age and physical and cognitive aging. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2020;75(3):504–511. doi: 10.1093/gerona/glz246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388–1396. doi: 10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breitling LP, Saum KU, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenetics. 2016;8(1). doi: 10.1186/s13148-016-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrory C, Fiorito G, Hernandez B, et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J Gerontol A Biol Sci Med Sci. Published online November 19, 2020. doi: 10.1093/gerona/glaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verschoor CP, Lin DTS, Kobor MS, et al. Epigenetic age is associated with baseline and 3-year change in frailty in the Canadian Longitudinal Study on Aging. Clin Epigenetics. 2021;13(1):1–10. doi: 10.1186/s13148-021-01150-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beydoun MA, Shaked D, Tajuddin SM, Weiss J, Evans MK, Zonderman AB. Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology. 2020;94(6):e613. doi: 10.1212/WNL.0000000000008756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degerman S, Josefsson M, Nordin Adolfsson A, et al. Maintained memory in aging is associated with young epigenetic age. Neurobiol Aging. 2017;55:167–171. doi: 10.1016/j.neurobiolaging.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 51.Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: Meta-analysis predicting time to death. Aging. 2016;8(9):1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8(1). doi: 10.1186/s13148-016-0228-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marioni RE, Harris SE, Shah S, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2016;45(2):424–432. doi: 10.1093/ije/dyw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dugué PA, Bassett JK, Joo JHE, et al. DNA methylation-based biological aging and cancer risk and survival: Pooled analysis of seven prospective studies. Int J Cancer. 2018;142(8):1611–1619. doi: 10.1002/ijc.31189 [DOI] [PubMed] [Google Scholar]
- 55.Clapp JD, Luta G, Small BJ, et al. The Impact of Using Different Reference Populations on Measurement of Breast Cancer-Related Cognitive Impairment Rates. Archives of Clinical Neuropsychology. 2018;33(8):956–963. doi: 10.1093/ARCLIN/ACX142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the Functional Assessment of Cancer Therapy Cognitive Scale with Hematopoetic Stem Cell Transplant Patients. J Pain Symptom Manage. 2007;33(1):13–23. doi: 10.1016/j.jpainsymman.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 57.Wagner L, Sweet J, Butt Z, Lai J, Cella D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. 2009;7(6):W32–W39. [Google Scholar]
- 58.Ware J, Kosinski M, Keller S. A 12-Item Short-Form Health Survey: Construction of Scales a... : Medical Care. Med Care. 1996;34(3):220–233. Accessed July 16, 2020. https://journals.lww.com/lww-medicalcare/Fulltext/1996/03000/A_12_Item_Short_Form_Health_Survey__Construction.3.aspx [DOI] [PubMed] [Google Scholar]
- 59.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 60.R Core Team. R: A language and environment for statistical computing. Published online 2022.
- 61.Scuric Z, Carroll JE, Bower JE, et al. Biomarkers of aging associated with past treatments in breast cancer survivors. NPJ Breast Cancer. 2017;3(1):1–7. doi: 10.1038/s41523-017-0050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carroll JE, van Dyk K, Bower JE, et al. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer. 2019;125(2):298–306. doi: 10.1002/cncr.31777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambatipudi S, Horvath S, Perrier F, et al. DNA methylome analysis identifies accelerated epigenetic ageing associated with postmenopausal breast cancer susceptibility. Eur J Cancer. 2017;75:299–307. doi: 10.1016/j.ejca.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kresovich JK, Xu Z, O’Brien KM, Weinberg CR, Sandler DP, Taylor JA. Methylation-Based Biological Age and Breast Cancer Risk. JNCI: Journal of the National Cancer Institute. 2019;111(10):1051–1058. doi: 10.1093/JNCI/DJZ020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Dyk K, Crespi CM, Petersen L, Ganz PA. Identifying Cancer-Related Cognitive Impairment Using the FACT-Cog Perceived Cognitive Impairment. JNCI Cancer Spectr. 2020;4(1). doi: 10.1093/JNCICS/PKZ099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll JE, Nakamura ZM, Small BJ, et al. Elevated C-Reactive Protein and Subsequent Patient-Reported Cognitive Problems in Older Breast Cancer Survivors: The Thinking and Living With Cancer Study. J Clin Oncol. Published online September 30, 2022:JCO2200406. doi: 10.1200/JCO.22.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim S, Nzegwu D, Wright ML. The Impact of Psychosocial Stress from Life Trauma and Racial Discrimination on Epigenetic Aging—A Systematic Review. Biol Res Nurs. 2022;24(2):202–215. doi: 10.1177/10998004211060561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE. Accelerated epigenetic aging mediates link between adverse childhood experiences and depressive symptoms in older adults: Results from the Health and Retirement Study. SSM Popul Health. 2022;17:101071. doi: 10.1016/j.ssmph.2022.101071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Polsky LR, Rentscher KE, Carroll JE. Stress-induced biological aging: A review and guide for research priorities. Brain Behav Immun. 2022;104:97–109. doi: 10.1016/J.BBI.2022.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE. Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proceedings of the National Academy of Sciences. 2022;119(25):e2202780119. doi: 10.1073/PNAS.2202780119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan J, Wrigglesworth J, Loong J, Fransquet PD, Woods RL. A Systematic Review and Meta-analysis of Environmental, Lifestyle, and Health Factors Associated With DNA Methylation Age. The Journals of Gerontology: Series A. 2020;75(3):481–494. doi: 10.1093/GERONA/GLZ099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oblak L, van der Zaag J, Higgins-Chen AT, Levine ME, Boks MP. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res Rev. 2021;69:101348. doi: 10.1016/J.ARR.2021.101348 [DOI] [PubMed] [Google Scholar]
- 73.Brody GH, Yu T, Chen E, Beach SRH, Miller GE. Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. J Child Psychol Psychiatry. 2016;57(5):566–574. doi: 10.1111/jcpp.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waziry R, Corcoran DL, Huffman KM, et al. Effect of Long-Term Caloric Restriction on DNA Methylation Measures of Biological Aging in Healthy Adults: CALERIE™ Trial Analysis. medRxiv. Published online 2021:2021.09.21.21263912. doi: 10.1101/2021.09.21.21263912 [DOI] [Google Scholar]
- 75.Fahy GM, Brooke RT, Watson JP, et al. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18(6). doi: 10.1111/ACEL.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of Vitamin D3 Supplementation on Epigenetic Aging in Overweight and Obese African Americans With Suboptimal Vitamin D Status: A Randomized Clinical Trial. The Journals of Gerontology: Series A. 2019;74(1):91–98. doi: 10.1093/GERONA/GLY223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sae-Lee C, Corsi S, Barrow TM, et al. Dietary Intervention Modifies DNA Methylation Age Assessed by the Epigenetic Clock. Mol Nutr Food Res. 2018;62(23):1800092. doi: 10.1002/MNFR.201800092 [DOI] [PubMed] [Google Scholar]
- 78.Browder KC, Reddy P, Yamamoto M, et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nature Aging 2022 2:3. 2022;2(3):243–253. doi: 10.1038/s43587-022-00183-2 [DOI] [PubMed] [Google Scholar]
- 79.Cole JJ, Robertson NA, Rather MI, et al. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. 2017;18(1):1–16. doi: 10.1186/S13059-017-1185-3/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for the Thinking and Living with Cancer (TLC) study used in this publication were supported by funding from the National Institutes of Health. The data are available for sharing under NIH-compliant TLC study agreements and FAIR principles (Findability, Accessibility, Interoperability, Reproducibility) for data access. Data access is provided via email requests to the study Principal Investigators and follows the TLC protocol. The SAS code and data for the analyses included in this paper are available upon request within the constraints of the TLC IRB requirements.


