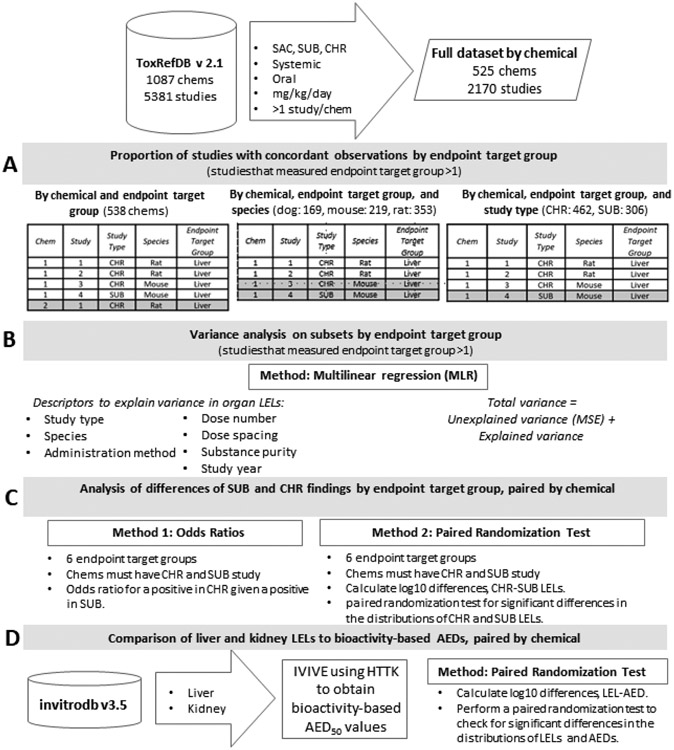
Figure 1. Experimental workflow.
The experimental workflow is described in parts A, B, C, and D. In A, different definitions of replicate studies for analysis of concordance are illustrated, where gray denotes the studies that would not be replicates for each grouping. In B, MLR employs descriptors of LEL to estimate explained variance by endpoint target group. In C, the differences between SUB and CHR organ-level findings are explored using odds ratios to describe the qualitative outcomes observed and paired randomization tests to identify quantitative differences. Finally, in D, using IVIVE, AEDs based on organ-relevant bioactivity assays in ToxCast are compared to LELs from ToxRefDB for kidney and liver.